MyMD Pharmaceuticals, Inc. - Common Stock (MYMD)
1.8200
+0.00 (0.00%)
NASDAQ · Last Trade: Nov 2nd, 11:41 PM EST

TNF Pharmaceuticals, Inc. (Nasdaq: TNFA) (formerly MyMD Pharmaceuticals, Inc.) (the “Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases and autoimmune and inflammatory conditions, today announced that its common stock, listed on the Nasdaq Capital Market, begins trading under the new ticker symbol, “TNFA,” effective before the market open today, July 24, 2024.
By TNF Pharmaceuticals, Inc. · Via Business Wire · July 24, 2024

TNF Pharmaceuticals, Inc., formerly MyMD Pharmaceuticals, Inc. (Nasdaq: MYMD) (the “Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases and autoimmune and inflammatory conditions, today announced a rebranding to the new name “TNF Pharmaceuticals, Inc.,” effective today. The Company’s common stock, listed on the Nasdaq Capital Market, will begin trading under the new stock symbol “TNFA,” effective before the market open on Wednesday, July 24, 2024.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · July 22, 2024

MyMD Pharmaceuticals, Inc. (Nasdaq: MYMD) (“MyMD” or the “Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases and autoimmune and inflammatory conditions, today announced the appointment of Mitchell Glass, M.D., a member of the board of directors of the Company, as president and chief medical officer. The Company also announced the appointment of Mr. Stephen Friscia, a veteran investor, to the board of directors.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · June 17, 2024
Intelligent Bio Solutions Inc. (NASDAQ: INBS) Advancing US FDA Process, Featured Among Top Health Care Movers
Intelligent Bio Solutions Inc. (NASDAQ: INBS), a medical technology company, offers rapid, intelligent, and non-invasive testing solutions. On May 29, the company announced its success in recruiting and starting the screening of subjects for its pharmacokinetic (PK) study. This study is a crucial step in Intelligent Bio Solutions' FDA 510(k) regulatory clearance process. The dedicated team worked diligently to ensure the study's design met FDA standards.
Via AB Newswire · May 31, 2024

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases, and autoimmune and inflammatory conditions, today announced that it has secured $7 million in commitments in two private placement funding rounds led by a strategic investor, PharmaCyte Biotech, Inc. (Nasdaq: PMCB), a clinical-stage biotechnology company developing cellular therapies for cancer and diabetes. An additional $7 million was raised from existing MyMD shareholders participating in the offerings. The closings of the two private placements are each subject to customary closing conditions and are both expected to occur on or around May 22, 2024.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · May 21, 2024
Ladenburg Thalmann, Esteemed Wall Street Long-Standing Stalwart Since 1876, Advocates ‘Buy’ for Intelligent Bio, Precision Bio, and More: VTAK, INBS, LASE, MYMD, RVSN
Investors are constantly searching for stocks that merit attention given recent developments. Yet, extensive research is necessary to uncover these stocks. This article offers insights into five stocks that may be worth monitoring currently.
Via AB Newswire · April 9, 2024

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, today announced that it received notice from The Nasdaq Stock Market LLC ("Nasdaq") on March 4, 2024 informing the Company that it has regained compliance with the minimum bid price requirement under Nasdaq Listing Rule 5550(a)(2) (“Listing Rule”) for continued listing on the Nasdaq Capital Market.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · March 5, 2024

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, announced today that it intends to effect a reverse stock split of its common stock at a ratio of 1 post-split share for every 30 pre-split shares. The reverse stock split will become effective at 4:05 p.m. Eastern Standard Time on Wednesday, February 14, 2024. MyMD’s common stock will continue to be traded on the Nasdaq Capital Market under the symbol MYMD and will begin trading on a split-adjusted basis when the market opens on Thursday, February 15, 2024. The new CUSIP number for MyMD’s common stock following the reverse stock split will be 62856X201.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · February 13, 2024

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, announced today that its Investigational New Drug (IND) application for a Phase 2 clinical trial of oral MYMD-1® as a treatment for rheumatoid arthritis (RA) was recently cleared by the U.S. Food and Drug Administration (FDA), and plans are underway for trial launch in the first quarter of 2024.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · December 6, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, today announced results from a preclinical study of its investigational cannabinoid Supera-CBD™, a novel, synthetic, non-toxic cannabidiol (CBD) analog. In the study, Supera-CBD targeted and quickly reduced inflammatory pain within 60 minutes, providing pain relief for up to five hours. Comparatively, traditional CBD had no effect on this type of pain in the same study.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · October 19, 2023
MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, announced that it plans to share information on the Company and its product pipeline, including an update on recent positive phase 2 study results for MYMD-1 in sarcopenia, at the upcoming BioFuture 2023 Meeting.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · October 4, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, announced today that the U.S. Food and Drug Administration (FDA) has accepted the Company’s Investigational New Drug Application (IND) to evaluate the safety, efficacy, pharmacodynamics and pharmacokinetics of oral TNF-α inhibitor MYMD-1® in patients with active rheumatoid arthritis (RA). The application was based on preclinical data showing that MYMD-1 significantly reduced swelling and other clinical arthritis measures compared to widely used RA therapy, Enbrel® (etanercept).1 The Company plans to initiate discussions with CRO vendor IQVIA on timing of a Phase 2 study in this indication.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · August 14, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or the “Company”), a clinical stage pharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, announced statistically significant positive topline Phase 2 results for its next generation Oral TNF-α inhibitor MYMD-1 in Sarcopenia/Age-Related Frailty earlier this week. In conjunction with its release, the company also announced it will hold a conference call today, August 2nd, at 4:30pm ET to discuss the results.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · August 2, 2023

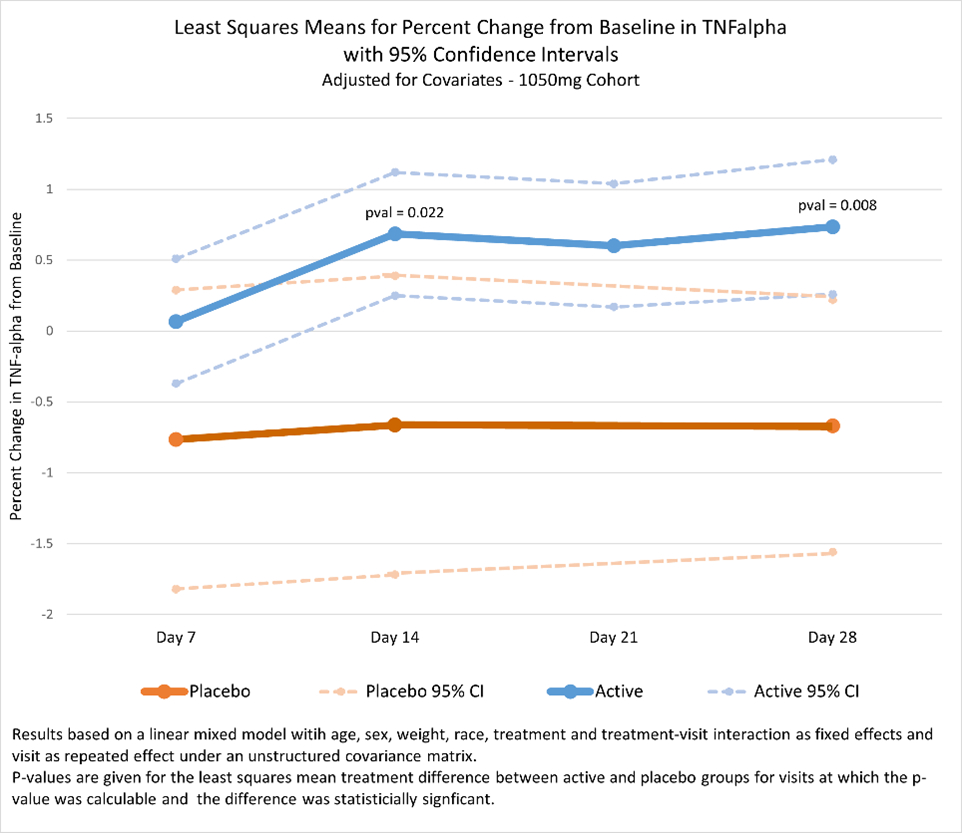
MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or the “Company”), a clinical stage pharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, today announced statistically significant positive topline results from its randomized Phase 2 study of oral TNF-α inhibitor, MYMD-1® in patients with chronic inflammation associated with sarcopenia, or age-related frailty. The study met its primary endpoints of significantly reducing chronic inflammatory markers in participants treated with MYMD-1. MYMD-1 has the potential to be the first drug approved by the United States Food and Drug Administration (FDA) for sarcopenia, an age-related decline in physical function which leads to greater risk of hospitalization, disability, and death.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · July 31, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, today announced a dosing update on its fully-funded Phase 2 clinical trial of lead drug candidate MYMD-1®, an orally available next-generation TNF-alpha inhibitor, as a therapy for chronic inflammation associated with sarcopenia and frailty (NCT05283486).
By MyMD Pharmaceuticals, Inc. · Via Business Wire · April 12, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, is presenting data from a preclinical study of investigational, oral TNF-α inhibitor MYMD-1® at the 2023 Society of Toxicology Annual Meeting (SOT) in Nashville, TN. Study results comparing MYMD-1 to placebo were very highly significant and showed MYMD-1 reduced histopathological changes and the severity of standard arthritis clinical trial measures.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · March 20, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, today announced that it has joined LOT Network, the international, non-profit community of companies working together to protect themselves against litigation brought on by patent assertion entities (PAEs, also known as “patent trolls”). MyMD’s joining the LOT (License on Transfer) Network’s community of 2,800+ companies is intended to enable MyMD to protect the interests of the Company against patent assertion entities.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · March 8, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, today announced that the U.S. Drug Enforcement Administration (DEA) has conducted a scientific review and determined that investigational cannabinoid Supera-CBD™ is not currently considered a controlled substance or listed chemical. The scientific review of the chemical structure of Supera-CBD was conducted in accordance with the Controlled Substances Act (CSA) and its governing regulations.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · March 2, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, announced that preclinical data from a study, conducted in partnership with Charles River Laboratories International, Inc., has been accepted for presentation at the upcoming Society of Toxicology (SOT) 2023 Annual Meeting.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · February 28, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, today announced that it has executed a securities purchase agreement to raise gross proceeds of approximately $15 million in a registered direct offering of convertible preferred stock and warrants with certain accredited and institutional investors. The offering is expected to close on February 23, 2023, subject to the satisfaction of customary closing conditions.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · February 21, 2023

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, is presenting data today at the 2022 British Society for Immunology (BSI) Congress in Liverpool, England. Results from preclinical and clinical studies showed that MYMD-1 was safe and well-tolerated in healthy subjects and significantly reduced inflammation in a mouse model compared to the widely used arthritis treatment etanercept (Enbrel®). These findings support the continued evaluation of MYMD-1® for autoimmune and inflammatory disorders.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · December 6, 2022

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, has been invited to present late-breaking data at the 2022 British Society for Immunology (BSI) Congress in Liverpool, England.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · November 17, 2022

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage pharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, today announced the publication of results from a Phase 1 study of oral tumor necrosis factor-alpha (TNF-α) inhibitor, MYMD-1® (Isomyosamine), in peer-reviewed journal, Drug Research. The randomized, double-blind, placebo-controlled study, intended to evaluate the safety, tolerability, and pharmacokinetic profile of MYMD-1 in healthy adults, found that single daily doses for 3 days and multiple daily doses for 6 days were safe and well tolerated in healthy subjects. The study used single daily doses of 150 mg, 300 mg, and 450 mg, respectively, and multiple daily doses of 600 mg. There were no new or unexpected safety findings and no clinically relevant or severe adverse events reported.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · November 14, 2022

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD” or “the Company”), a clinical stage biopharmaceutical company developing groundbreaking therapies for the treatment of serious and debilitating autoimmune and inflammatory diseases, announced a dosing update on its fully-funded Phase 2 clinical trial of lead drug candidate MYMD-1®, an orally available next-generation TNF-alpha inhibitor, as a therapy for chronic inflammation associated with sarcopenia and frailty.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · September 20, 2022

MyMD Pharmaceuticals, Inc.® (Nasdaq: MYMD) (“MyMD”), a clinical stage pharmaceutical company committed to developing novel therapies for age-related diseases, autoimmune and inflammatory conditions, announced today that it received a decision to grant from the European Patent Office in its application no. EP 3755318 A1. The allowed claims cover MyMD’s Supera-CBD™ compound as a new molecular entity and pharmaceutical formulations containing the compound.
By MyMD Pharmaceuticals, Inc. · Via Business Wire · August 30, 2022
