Articles from Vivani Medical, Inc.

Vivani Medical, Inc. (NASDAQ: VANI) (“Vivani” or the “Company”), a clinical-stage biopharmaceutical company developing miniature, ultra long-acting drug implants, announced today that it will present at the Emerging Growth Conference on March 27, 2025.
By Vivani Medical, Inc. · Via Business Wire · March 24, 2025

Vivani Medical, Inc. (NASDAQ: VANI) (“Vivani” or the “Company”), a clinical-stage biopharmaceutical company developing miniature, ultra long-acting drug implants, today announced the successful administration of its first GLP-1 (exenatide) implant in the LIBERATE-1™ clinical trial. This milestone marks a critical step toward addressing one of healthcare’s most pressing challenges: medication adherence in metabolic diseases including chronic weight management and type 2 diabetes. The Company also announced full enrollment in the LIBERATE-1 study, which was achieved in just four weeks after enrollment of the first subject, signaling early potential interest for this six-month, subdermal GLP-1 implant and reaffirming previous estimates that top-line results should be available in mid-2025.
By Vivani Medical, Inc. · Via Business Wire · March 13, 2025

Vivani Medical, Inc. (NASDAQ: VANI) (“Vivani” or the “Company”), a clinical-stage biopharmaceutical company developing miniature, ultra long-acting drug implants, today announced that it intends to spin off Cortigent, Inc., a division that develops brain implant devices to help people recover critical body functions, as an independent publicly-traded company. The strategic goal of this transaction is to create two focused companies dedicated to driving current and future value in their respective therapeutic areas of expertise.
By Vivani Medical, Inc. · Via Business Wire · March 12, 2025

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative biopharmaceutical company developing novel, ultra long-acting drug implants, today announced that screening and enrollment of the first-in-human clinical trial, known as LIBERATE-1™, has been initiated at two centers in Australia to investigate the safety, tolerability and full pharmacokinetic profile of an exenatide implant. This study is the first clinical application of the Company’s proprietary NanoPortal™ drug implant technology.
By Vivani Medical, Inc. · Via Business Wire · December 19, 2024

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an innovative biopharmaceutical company developing miniaturized, ultra long-acting drug implants, announced today that CEO Adam Mendelsohn, Ph.D., will present and participate in a panel discussion at the Innovation in Obesity Therapeutics Summit West Coast, taking place in San Diego, California, from December 10-12, 2024.
By Vivani Medical, Inc. · Via Business Wire · December 4, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-acting drug implants, today reported financial results for the third quarter ended September 30, 2024, and provided a business update.
By Vivani Medical, Inc. · Via Business Wire · November 13, 2024

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an innovative biopharmaceutical company developing miniaturized, ultra long-acting drug implants, announced today that it will present at two upcoming events in October: (1) Partnership Opportunities in Drug Delivery (PODD), taking place in Boston on October 28 and 29, 2024 and (2) the ThinkEquity Conference, taking place in New York on October 30, 2024.
By Vivani Medical, Inc. · Via Business Wire · October 22, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative, biopharmaceutical company developing novel, ultra long-acting drug implants, today announced that the Bellberry Human Research Ethics Committee (“HREC”) has approved and the Therapeutic Goods Administration (“TGA”) in Australia has formally acknowledged a first in human clinical trial of the Company’s miniature, subdermal GLP-1 (exenatide) implant in obese and overweight subjects. This clinical trial, known as LIBERATE-1™, will investigate the safety, tolerability and full pharmacokinetic profile of an exenatide implant and represents the first clinical application of the Company’s proprietary NanoPortal™ drug implant technology.
By Vivani Medical, Inc. · Via Business Wire · September 26, 2024

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an innovative biopharmaceutical company developing miniaturized, ultra long-acting drug implants, announced today that it will present at the Emerging Growth Conference on September 25, 2024.
By Vivani Medical, Inc. · Via Business Wire · September 23, 2024
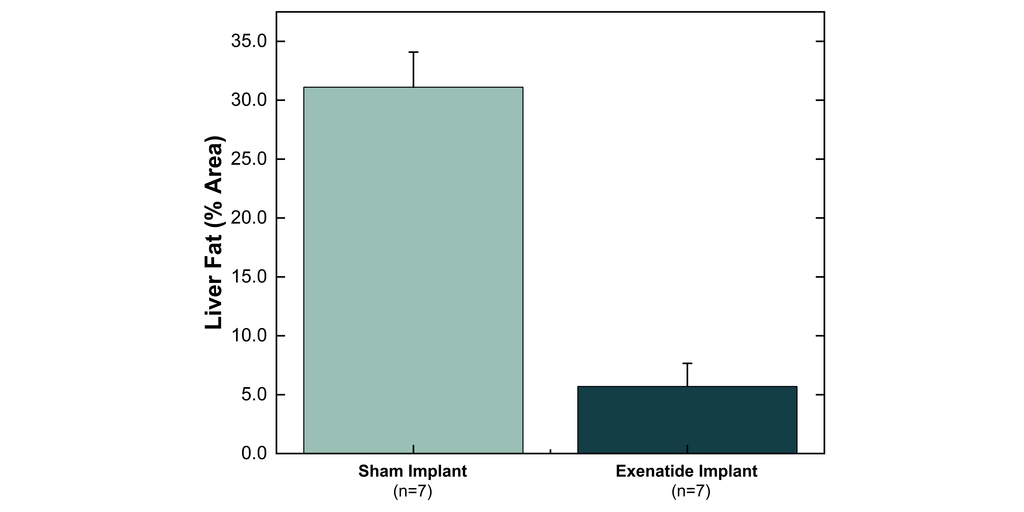
Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative biopharmaceutical company developing miniature, ultra long-acting, subdermal drug implants, today reported positive preclinical liver fat results with its exenatide implant.
By Vivani Medical, Inc. · Via Business Wire · September 4, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative biopharmaceutical company developing miniature, ultra long-acting, subdermal drug implants, today announced its plans to participate in the H.C. Wainwright 26th Annual Global Investment Conference taking place September 9-11, 2024 in New York, New York.
By Vivani Medical, Inc. · Via Business Wire · August 28, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-acting drug implants, today reported financial results for the second quarter ended June 30, 2024, and provided a business update.
By Vivani Medical, Inc. · Via Business Wire · August 13, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative, biopharmaceutical company developing novel, long-term drug implants, today announced that it expects to initiate the first clinical study in the NPM-115 program in the fourth quarter of 2024 in Australia, pending regulatory clearance in that country. The NPM-115 clinical program will evaluate the investigational 6-month GLP-1 implant for chronic weight management in patients who are either obese or overweight with a related comorbidity.
By Vivani Medical, Inc. · Via Business Wire · July 11, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative, biopharmaceutical company developing novel, long-term drug implants, today announced the U.S. Food and Drug Administration has cleared the Investigational New Drug Application (“IND”) and lifted the clinical hold on NPM-119 to allow initiation of LIBERATE-1™, a Phase 1 clinical trial to assess the safety, tolerability and pharmacokinetics of NPM-119 (exenatide), the Company’s miniature, six-month GLP-1 implant in development for the treatment of type 2 diabetes.
By Vivani Medical, Inc. · Via Business Wire · June 13, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-term drug implants including its lead asset NPM-115 for chronic weight management in obese or overweight human patients with one or more risk factors, today announced the publication of positive proof-of-concept weight loss data with OKV-119, a miniature, subdermal, exenatide drug implant designed to treat feline obesity and diabetes, in the peer-reviewed BMC Veterinary Research. The Company has licensed the rights of its exenatide delivery system, supported by the proprietary NanoPortal™ platform, to development partner, Okava Pharmaceuticals, which is studying OKV-119 for the treatment of pre-diabetes, diabetes and obesity in companion felines.
By Vivani Medical, Inc. · Via Business Wire · May 28, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-term drug implants including its lead asset NPM-115 for chronic weight management in obese or overweight patients with one or more risk factors, today reported financial results for the first quarter ended March 31, 2024, and provided a business update.
By Vivani Medical, Inc. · Via Business Wire · May 13, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-term drug implants including its lead asset NPM-115 for chronic weight management in obese or overweight patients with one or more risk factors, today announced that it will present at the TIDES Conference 2024 at 4:30 p.m. ET at the Hynes Convention Center in Boston on Friday, May 17.
By Vivani Medical, Inc. · Via Business Wire · May 9, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-term drug implants including its lead asset NPM-115 for chronic weight management in obese or overweight patients with one or more risk factors, today reported financial results for the fourth quarter and full year ended December 31, 2023, and provided a business update.
By Vivani Medical, Inc. · Via Business Wire · March 26, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative, preclinical-stage biopharmaceutical company developing novel, long-term drug implants, today announced the appointment of long-time industry veteran Daniel Bradbury to its Board of Directors.
By Vivani Medical, Inc. · Via Business Wire · March 6, 2024

Vivani Medical, Inc. (Nasdaq: VANI) ("Vivani" or the "Company"), an innovative, preclinical-stage biopharmaceutical company developing novel, long-term drug implants, today announced that it has entered into a securities purchase agreement with an institutional investor to purchase 3,947,368 shares of common stock and warrants to purchase up to an aggregate of 3,947,368 shares of common stock at a purchase price of $3.80 per share and accompanying warrant in a registered direct offering. The warrants have an exercise price of $3.80 per share, are exercisable immediately upon issuance, and will expire three years following the date of issuance.
By Vivani Medical, Inc. · Via Business Wire · March 1, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative, preclinical-stage biopharmaceutical company developing novel, long-term drug implants, today announced positive preclinical data on weight loss effects for NPM-115, the Company’s miniature, twice-yearly, exenatide implant under development for the treatment of chronic weight management. The Company also disclosed that semaglutide is the active pharmaceutical ingredient in NPM-139, a miniature, subdermal GLP-1 implant in development for chronic weight management, with the added potential benefit of once-yearly administration. These developments are part of a strategic shift to prioritize the Company’s obesity implants based on emerging data regarding the potential for high-dose GLP-1 products to improve health outcomes for obese and overweight patients.
By Vivani Medical, Inc. · Via Business Wire · February 28, 2024

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative, preclinical-stage biopharmaceutical company developing novel, long-term drug implants, today reported financial results for the third quarter of 2023 and provided a business update.
By Vivani Medical, Inc. · Via Business Wire · November 14, 2023

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an innovative, preclinical-stage biopharmaceutical company that develops novel, long-term drug implants, announced today that it will present at the BIO Investor Forum at 2:00 p.m. PDT at the Hilton San Francisco Union Square on October 18, 2023.
By Vivani Medical, Inc. · Via Business Wire · October 16, 2023

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an innovative, preclinical-stage biopharmaceutical company that develops novel, long-term drug implants, announced today that it will present at the upcoming Annual Boulder Peptide Symposium at 11:20 am PST at the Napa Valley Marriott Hotel and Spa in Napa, California on September 19, 2023.
By Vivani Medical, Inc. · Via Business Wire · September 7, 2023

Vivani Medical, Inc. (NASDAQ: VANI), an innovative, preclinical-stage biopharmaceutical company developing novel, long-term drug implants, announced today that its wholly-owned subsidiary Cortigent, Inc., a company pioneering targeted neurostimulation technology to recover critical body functions, will present the Early Feasibility Study (EFS) results for the Orion® Visual Cortical Prosthesis System at The Eye & The Chip: 13th World Research Congress on Artificial Vision in Southfield, Michigan on October 8-10, 2023.
By Vivani Medical, Inc. · Via Business Wire · September 5, 2023

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative, preclinical-stage biopharmaceutical company developing novel, long-term drug implants, today reported financial results for the second quarter of 2023 and provided a business update.
By Vivani Medical, Inc. · Via Business Wire · August 14, 2023

Vivani Medical, Inc. (NASDAQ: VANI), an innovative, near-clinical stage biopharmaceutical company that is developing novel, miniature, long-term therapeutic implants, announced today that its wholly-owned subsidiary Cortigent, Inc., a company pioneering neurostimulation to recover critical body function, has completed the fifth year of its Early Feasibility Study (EFS) of profoundly blind patients implanted with the Orion® Visual Cortical Prosthesis System. The National Institutes of Health - funded Orion EFS commenced in 2017 and enrolled six subjects at two universities, the University of California, Los Angeles (UCLA) and Baylor College of Medicine. Five years after implantation of the Orion device on the surface of the visual cortex area of the brain, the three subjects who are still participating in the study have reported no device malfunctions and continue to be able to use their systems at home and in their communities. In terms of safety, a single serious adverse event (SAE) was reported during initial testing in the first three months after implantation which resolved quickly and resulted in no permanent harm. No SAEs have been reported since then.
By Vivani Medical, Inc. · Via Business Wire · July 11, 2023

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an innovative, near clinical-stage biopharmaceutical company that develops novel, miniature, long-term therapeutic implants, announced today that Company CEO Adam Mendelsohn, Ph.D. has been invited to participate at the Healthcare Virtual Conference Part II, presented by Maxim Group LLC and hosted by M-Vest. Dr. Mendelsohn will participate in the panel discussion entitled “Oh! Oh! Ozempic - Metabolics are in Focus” on June 20 at 11:00 am ET.
By Vivani Medical, Inc. · Via Business Wire · June 14, 2023

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-term drug implants, including lead product NPM-119 for the treatment of patients with type 2 diabetes and/or obesity, today reported financial results for the first quarter and provided a business update. NPM-119 is a preclinical stage, 6-month, GLP-1 implant using Vivani’s proprietary NanoPortal™ implant technology.
By Vivani Medical, Inc. · Via Business Wire · May 15, 2023

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an emerging biopharmaceutical company which develops miniaturized, subdermal implants today announced that the Company received notice from the National Institutes of Health (“NIH”) of approval of the year 5 funding for the Early Feasibility Clinical Study of the Orion Visual Cortical Prosthesis being conducted by its newly formed subsidiary, Cortigent, Inc. This represents the final $1 million funding of the original $6.4 million five-year grant award.
By Vivani Medical, Inc. · Via Business Wire · April 4, 2023

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-term drug implants, including lead product candidate NPM-119 for the treatment of patients with type 2 diabetes, today reported financial results for the fourth quarter and full year ended December 31, 2022, and provided a business update.
By Vivani Medical, Inc. · Via Business Wire · March 31, 2023

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an innovative, clinical-stage biopharmaceutical company that develops novel, long-term therapeutic implants, today announced the filing of a Registration Statement on Form S-1 with the U.S. Securities and Exchange Commission (“SEC”) for the proposed initial public offering of Cortigent, Inc. (“Cortigent”). Cortigent, currently a wholly-owned subsidiary of Vivani, was formed for the purpose of advancing the business of Vivani’s neuromodulation division and will continue to be controlled by Vivani following the initial public offering. Cortigent is led by its Chief Executive Officer, Jonathan Adams.
By Vivani Medical, Inc. · Via Business Wire · March 21, 2023

Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a biopharmaceutical company developing miniaturized, long-term drug implants including lead asset NPM-119 for the treatment of patients with type 2 diabetes, today announced financial results for the third quarter ending September 30, 2022 and provides business updates.
By Vivani Medical, Inc. · Via Business Wire · November 14, 2022

Vivani Medical, Inc. (NASDAQ: VANI) (the “Company” or “Vivani”), an innovative, clinical-stage biopharmaceutical company that develops novel, long-term therapeutic implants, announced today that it will present at the upcoming ThinkEquity investor conference at 11:00 am EDT at the Mandarin Oriental in New York on October 26, 2022.
By Vivani Medical, Inc. · Via Business Wire · October 17, 2022

Vivani Medical, Inc., formerly Second Sight Medical, Inc., (NASDAQ: VANI) (the “Company” or “Vivani”) announced that trading of the Company’s common stock on The Nasdaq Capital Market under the symbol “VANI” will commence today.
By Vivani Medical, Inc. · Via Business Wire · August 31, 2022
