Articles from Vertex Pharmaceuticals Incorporated

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in TD Cowen’s 46th Annual Health Care Conference. Reshma Kewalramani, President and Chief Executive Officer will participate in a fireside chat on Tuesday, March 3, 2026, at 9:10 a.m. ET.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · February 17, 2026

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the fourth quarter and full year ended December 31, 2025, and provided its full year 2026 financial guidance.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · February 12, 2026

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its fourth quarter and full year 2025 financial results on Thursday, February 12, 2026, after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals Fourth Quarter 2025 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 20, 2026

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced business and program updates ahead of upcoming investor meetings in January, including the company’s scheduled webcast from the 44th annual J.P. Morgan Healthcare Conference on Monday, January 12, 2026, at 5:15 p.m. ET/2:15 p.m. PT.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 11, 2026

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that Dr. Reshma Kewalramani, Chief Executive Officer and President, will present at the 44th Annual J.P. Morgan Healthcare Conference on Monday, January 12, 2026, at 5:15 p.m. ET/2:15 p.m. PT.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 22, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced data from multiple studies demonstrating the clinical benefits of CASGEVY® (exagamglogene autotemcel) in people ages 5 years and older living with severe sickle cell disease (SCD) or transfusion-dependent beta thalassemia (TDT). The results, including the first presentation of clinical data from pivotal studies in children ages 5-11 years, and longer-term data from the pivotal studies of people with severe SCD and TDT ages 12 years and older, will be presented at the American Society of Hematology (ASH) Annual Meeting. CASGEVY is currently approved for eligible people ages 12 years and older with SCD or TDT in the United States, Great Britain, the European Union, the Kingdom of Saudi Arabia, the Kingdom of Bahrain, Kuwait, Qatar, Canada, Switzerland and the United Arab Emirates.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 6, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in Citi’s 2025 Global Healthcare Conference. Charlie Wagner, Chief Operating and Financial Officer, and David Altshuler, Chief Scientific Officer, will participate in a fireside chat on Wednesday, December 3, 2025, at 9:00 a.m. ET.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · November 19, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced updated data for povetacicept (pove) in IgA nephropathy (IgAN) and primary membranous nephropathy (pMN) from the ongoing RUBY-3 trial at the American Society of Nephrology (ASN) Kidney Week 2025 in Houston, Texas. Pove is an investigational recombinant fusion protein therapeutic and dual inhibitor of the BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand) cytokines. Pove is the only BAFF+APRIL inhibitor in pivotal trials for multiple kidney diseases.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · November 8, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the third quarter ended September 30, 2025, and refined full year 2025 financial guidance.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · November 3, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in the UBS Global Healthcare Conference. Reshma Kewalramani, President and Chief Executive Officer will participate in a fireside chat on Tuesday, November 11, 2025, at 10:15 a.m. ET.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · October 27, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced the presentation of multiple abstracts demonstrating the clinical benefits of treatment with CFTR modulators, including the Company’s most recently approved medicine, ALYFTREK® (vanzacaftor/tezacaftor/deutivacaftor), at the North American Cystic Fibrosis Conference (NACFC) held from October 22-25 in Seattle, Washington.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · October 23, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced several important updates across its development program for povetacicept (pove), an investigational recombinant fusion protein therapeutic and dual antagonist of the BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand) cytokines. Pove has demonstrated best-in-class potential in IgA nephropathy (IgAN) and primary membranous nephropathy (pMN) and has pipeline-in-a-product potential across a range of B cell-mediated diseases. Pove is the only BAFF+APRIL inhibitor in pivotal trials for multiple kidney diseases.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · October 17, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its third quarter 2025 financial results on Monday, November 3, 2025, after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals Third Quarter 2025 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · October 6, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced several important advancements across its programs in immunoglobulin A Nephropathy (IgAN), APOL1-mediated kidney disease (AMKD) and autosomal dominant polycystic kidney disease (ADPKD). These updates represent significant progress toward reaching the Company’s goal of bringing forward first-in-class or best-in-class therapies that target the underlying cause of these serious kidney diseases.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · September 25, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the company has partnered with basketball world champion Jayson Tatum to raise awareness of JOURNAVX® (suzetrigine), a prescription non-opioid medicine for the treatment of moderate-to-severe acute pain in adults and the first new class of pain medicine approved in more than 20 years.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · September 23, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that Senior Vice President Paul Negulescu has been named as one of the winners of this year’s Lasker~DeBakey Clinical Medical Research Award for his role “in the development of a novel, life-saving treatment for cystic fibrosis (CF) — namely, a triple-drug combination therapy, TRIKAFTA®, that has helped countless people with this genetic disease.” Dr. Negulescu is one of three awardees, alongside Jesús (Tito) González, a former Vertex scientist, and Michael Welsh, Professor of Internal Medicine-Pulmonary, Critical Care and Occupational Medicine, University of Iowa.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · September 11, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in three upcoming investor conferences.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · August 20, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the second quarter ended June 30, 2025, and reiterated full year 2025 financial guidance.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · August 4, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced topline results from its recently completed Phase 2, randomized, double-blind, placebo-controlled dose-ranging study evaluating the safety and efficacy of its investigational selective NaV1.8 pain signal inhibitor, VX-993, in treating acute pain after bunionectomy surgery. Treatment with VX-993 did not result in a statistically significant improvement on the primary endpoint of the time-weighted Sum of the Pain Intensity Difference from 0 to 48 hours (SPID48) compared to placebo.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · August 4, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that it has been named a 2025 Breakthroughs Innovation Celebration winner by Premier, Inc., a leading health care improvement company. The honor recognizes JOURNAVX™ (suzetrigine), an oral, non-opioid, highly selective NaV1.8 pain signal inhibitor for the treatment of adults with moderate-to-severe acute pain, as a groundbreaking health care technology.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · July 14, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its second quarter 2025 financial results on Monday, August 4, 2025 after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals Second Quarter 2025 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · July 8, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) and Ono Pharmaceutical Co., Ltd. (OTCMKTS: OPHLY) today announced an exclusive collaboration and license agreement for the development and commercialization of Vertex’s povetacicept in Japan and South Korea. Povetacicept is a recombinant fusion protein therapeutic and dual antagonist of the BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand) cytokines with best-in-class potential being studied for the treatment of immunoglobulin A nephropathy (IgAN), primary membranous nephropathy (pMN) and other B cell-mediated diseases.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · June 23, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced simultaneous presentation and publication of updated data from the Phase 1/2 portion of the Phase 1/2/3 FORWARD-101 clinical trial of zimislecel (VX-880), an investigational stem cell-derived, fully differentiated islet cell therapy, in people with type 1 diabetes (T1D) with impaired hypoglycemic awareness and severe hypoglycemic events (SHEs). The data were featured in an oral presentation at the American Diabetes Association (ADA) annual conference in Chicago as part of the symposium, “Innovation and Progress in Stem Cell-Derived Islet-Cell Replacement Therapy,” from 6:15-6:30 p.m. CT (abstract 2025-A-1921) and published online by the New England Journal of Medicine.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · June 20, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced data across multiple studies demonstrating positive clinical and quality of life benefits of treatment with CFTR modulators and, in particular, ALYFTREK® (vanzacaftor/tezacaftor/deutivacaftor), which is approved in the United States and United Kingdom and is currently under review with health authorities in the EU, Canada, Australia, New Zealand and Switzerland. These data were presented at this year’s European Cystic Fibrosis Society’s (ECFS) 48th European Cystic Fibrosis Conference held June 4-7, 2025, in Milan, Italy.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · June 6, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in three upcoming investor conferences.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · May 15, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the first quarter ended March 31, 2025, and raised the low end of its total revenue guidance range by $100 million, from $11.75 billion to $12 billion to a new range of $11.85 billion to $12 billion.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · May 5, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that Paul Negulescu, Ph.D. Senior Vice President, Vertex has been awarded the 2025 Canada Gairdner International Award “for pioneering research into the cellular and molecular mechanisms underlying the genetic disease cystic fibrosis, leading to the development of transformative drug therapies based on these mechanisms, thereby improving and saving countless lives.” Negulescu shares the award with Michael J. Welsh, M.D., University of Iowa.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · April 11, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its first quarter 2025 financial results on Monday, May 5, 2025 after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals First Quarter 2025 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · April 7, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced several updates on the Company’s type 1 diabetes (T1D) portfolio.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · March 28, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in three upcoming investor conferences.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · February 18, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the fourth quarter and full year ended December 31, 2024, and provided its full year 2025 financial guidance.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · February 10, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the U.S. Food and Drug Administration (FDA) has approved JOURNAVX™ (suzetrigine), an oral, non-opioid, highly selective NaV1.8 pain signal inhibitor for the treatment of adults with moderate-to-severe acute pain. JOURNAVX is an effective, well-tolerated medicine without evidence of addictive potential indicated for use across all types of moderate-to-severe acute pain.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 30, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its fourth quarter and full year 2024 financial results on Monday, February 10, 2025 after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals Fourth Quarter 2024 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 27, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced multiple program updates ahead of upcoming investor meetings in January, including the company’s scheduled webcast from the 43rd Annual J.P. Morgan Healthcare Conference on Monday, January 13, 2025, at 10:30 a.m. ET/7:30 a.m. PT.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 12, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) and Zai Lab Limited (Nasdaq: ZLAB; HKEX: 9688) today announced an exclusive collaboration and license agreement for the development and commercialization of Vertex’s povetacicept (pove) in mainland China, Hong Kong SAR, Macau SAR, Taiwan region and Singapore (the licensed territory). Pove is a recombinant fusion protein therapeutic and dual antagonist of BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand) with best-in-class potential being studied for the treatment of Immunoglobulin A nephropathy (IgAN) and other B cell-mediated diseases.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 10, 2025

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that Dr. Reshma Kewalramani, Chief Executive Officer and President, will present at the 43rd Annual J.P. Morgan Healthcare Conference on Monday, January 13, 2025 at 10:30 a.m. ET/7:30 a.m. PT.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 23, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the U.S. Food and Drug Administration (FDA) has approved ALYFTREK (vanzacaftor/tezacaftor/deutivacaftor), a once-daily next-in-class triple combination cystic fibrosis transmembrane conductance regulator (CFTR) modulator for the treatment of cystic fibrosis (CF) in people 6 years and older who have at least one F508del mutation or another mutation in the CFTR gene that is responsive to ALYFTREK. See below for Important Safety Information, including a Boxed Warning.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 20, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced the U.S. Food and Drug Administration (FDA) has approved the expanded use of TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor) for the treatment of people with cystic fibrosis (CF) ages 2 and older who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene or a mutation that is responsive to TRIKAFTA based on clinical and/or in vitro data. In addition, safety information on liver injury and liver failure has been updated from warnings and precautions to a boxed warning. With this approval, 94 additional non-F508del CFTR mutations have been added to the TRIKAFTA label, and approximately 300 additional people with CF in the U.S. are now eligible for a medicine to treat the underlying cause of their disease for the first time.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 20, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced results from its Phase 2 study of suzetrigine, an investigational, oral, highly selective NaV1.8 pain signal inhibitor in people with painful lumbosacral radiculopathy (LSR). The study met its primary endpoint with statistically significant and clinically meaningful reduction in pain on the numeric pain rating scale (NPRS).
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 19, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced longer-term data for CASGEVY™ (exagamglogene autotemcel) from global clinical trials in people with severe sickle cell disease (SCD) or transfusion-dependent beta thalassemia (TDT). CASGEVY is the first and only approved CRISPR/Cas9 gene-edited therapy.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 8, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the third quarter ended September 30, 2024, and raised its full-year product revenue guidance to $10.8 billion to $10.9 billion.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · November 4, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in two upcoming investor conferences.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · October 29, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today provided updates on multiple kidney diseases in its pipeline including IgA nephropathy (IgAN), primary membranous nephropathy (pMN), and APOL1-mediated kidney disease (AMKD). These updates demonstrate the transformative potential of Vertex’s investigational therapies in multiple serious kidney diseases, and include positive new data on povetacicept, a dual inhibitor of the BAFF and APRIL pathways, in IgAN and pMN, presented at the American Society of Nephrology’s (ASN) Kidney Week Congress on October 23-27 in San Diego, California.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · October 25, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the company will present its pivotal Phase 3 data on suzetrigine, an investigational, oral, highly selective NaV1.8 pain signal inhibitor for the treatment of moderate-to-severe acute pain, at the annual meeting of the American Society of Anesthesiologists (ASA), taking place from October 18-22, 2024 in Philadelphia, Pennsylvania.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · October 18, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its third quarter 2024 financial results on Monday, November 4, 2024 after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals Third Quarter 2024 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · October 10, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced the first accepted medical presentations of the Phase 3 data on the investigational once daily vanzacaftor/tezacaftor/deutivacaftor (“vanza triple”) — the potential next-in-class triple combination medicine — will take place at the North American Cystic Fibrosis Conference (NACFC). Vertex also announced presentations describing long-term outcomes in people with cystic fibrosis (CF) ages 2 to 11 years taking TRIKAFTA®, demonstrating consistent and sustained improvements across multiple measures of disease.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · September 26, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in two upcoming investor conferences.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · August 22, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the second quarter ended June 30, 2024, and raised its full year product revenue guidance to $10.65 to $10.85 billion.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · August 1, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) submission for suzetrigine, an investigational, oral, selective NaV1.8 pain signal inhibitor to treat moderate-to-severe acute pain. Suzetrigine has the potential to be the first new class of medicine to treat acute pain in over twenty years.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · July 30, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) for investigational once-daily vanzacaftor/tezacaftor/deutivacaftor triple combination therapy (vanza triple) for people living with cystic fibrosis (CF) ages 6 years and older who have at least one F508del mutation or another responsive mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene responsive to the vanza triple. Vertex used a priority review voucher for this submission reducing the review time from 10 months to 6 months, resulting in a Prescription Drug User Fee Act (PDUFA) target action date of January 2, 2025.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · July 2, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its second quarter 2024 financial results on Thursday, August 1, 2024 after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals Second Quarter 2024 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · July 1, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today presented new data from its Phase 1/2 clinical trial of VX-880, an investigational stem cell-derived, fully differentiated islet cell therapy, in people with type 1 diabetes (T1D) with impaired hypoglycemic awareness and severe hypoglycemic events (SHEs). These updated data on 12 patients who received the full dose as a single infusion in Parts B and C of the trial are consistent with previously reported positive results in the VX-880 trial and reinforce the transformative potential of this therapy.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · June 21, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced longer-term data for CASGEVY™ (exagamglogene autotemcel [exa-cel]) from global clinical trials in people with severe sickle cell disease (SCD) or transfusion-dependent beta thalassemia (TDT). The results, presented at the annual European Hematology Association (EHA) Congress, confirm the transformative, consistent and durable clinical benefits of CASGEVY over time. CASGEVY is the first and only approved CRISPR-based gene-editing therapy.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · June 14, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that data on TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor), also known in the European Union and in the U.K. as KAFTRIO® (ivacaftor/tezacaftor/elexacaftor) in combination with ivacaftor, were presented at this year’s European Cystic Fibrosis Society’s (ECFS) 47th European Cystic Fibrosis Conference held June 5-8, 2024, in Glasgow, Scotland.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · June 7, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced management participation in two upcoming investor conferences.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · May 22, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that Jennifer Schneider, M.D., M.S., has been elected to its Board of Directors as an independent director. Dr. Schneider has more than two decades of experience in the health care industry as a physician, scientist and health care executive. She co-founded and serves as Chief Executive Officer of Homeward Health, a company committed to rearchitecting the delivery of health and care for nearly 60 million Americans living in rural America. Prior to founding Homeward in 2022, Dr. Schneider served as President and Chief Medical Officer of Livongo Health, a biotechnology company serving people with chronic conditions including diabetes, hypertension, obesity and behavioral health issues. While there, she led the company through the largest consumer digital health Initial Public Offering in history and the industry’s largest merger between Livongo and Teladoc Health. She also served as Chief Medical Officer of Castlight Health, a health care navigation company for self-insured companies.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · May 15, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the first quarter ended March 31, 2024, and reiterated full year 2024 financial guidance.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · May 6, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced important advancements across its suzetrigine pain program, which has the potential to be the first new class of medicine for acute and neuropathic pain in more than two decades. Suzetrigine is an oral selective NaV1.8 pain signal inhibitor (formerly known as VX-548).
By Vertex Pharmaceuticals Incorporated · Via Business Wire · April 18, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its first quarter 2024 financial results on Monday, May 6, 2024 after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals First Quarter 2024 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · April 9, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that inaxaplin (VX-147) has advanced into the Phase 3 portion of the global Phase 2/3 pivotal clinical trial in APOL1-mediated kidney disease (AMKD), where a 45 mg once daily oral dose will be compared to placebo, on top of standard of care. The clinical trial is designed to assess the impact of inaxaplin on kidney function and proteinuria for people living with proteinuric kidney disease mediated by two variants in the APOL1 gene, known as AMKD. In addition, the trial has been expanded to include adolescents with AMKD ages 10 to 17 years.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · April 1, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug Application (IND) for VX-407, an investigational first-in-class small molecule corrector that targets the underlying cause of autosomal dominant polycystic kidney disease (ADPKD) in patients with a subset of PKD1 genetic variants. ADPKD is the most common inherited kidney disease, with an estimated 250,000 people in the U.S. and Europe living with ADPKD; however, there are no treatments currently available that address the underlying causal biology of the disease.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · March 21, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that Charles Wagner, Executive Vice President and Chief Financial Officer, and David Altshuler, M.D., Ph.D., Executive Vice President, Global Research, and Chief Scientific Officer, will participate in two upcoming investor conferences:
By Vertex Pharmaceuticals Incorporated · Via Business Wire · February 20, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) announced today that the European Commission has granted conditional marketing authorization to CASGEVY™ (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 gene-edited therapy. CASGEVY is approved for the treatment of patients who are 12 years of age and older with severe sickle cell disease (SCD) characterized by recurrent vaso-occlusive crises (VOCs) or transfusion-dependent beta thalassemia (TDT), for whom hematopoietic stem cell (HSC) transplantation is appropriate and a human leukocyte antigen matched related HSC donor is not available.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · February 13, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today reported consolidated financial results for the fourth quarter and full year ended December 31, 2023 and provided full year 2024 financial guidance.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · February 5, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced positive results from its once-daily vanzacaftor/tezacaftor/deutivacaftor (the “vanza triple”) program, the most comprehensive Phase 3 pivotal program ever conducted by Vertex for the treatment of cystic fibrosis (CF), a progressive, multi-organ disease caused by dysfunction of the CFTR protein. The Phase 3 program included two randomized, double-blind, active-controlled, 52-week trials, SKYLINE 102 and SKYLINE 103, evaluating the efficacy of vanzacaftor (20 mg)/tezacaftor (100 mg)/deutivacaftor (250 mg) once daily in people with CF ages 12 years and older who have at least one F508del mutation or a mutation responsive to triple combination CFTR modulators (CFTRm), compared to TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor). A third Phase 3 single-arm, 24‑week, open-label study, RIDGELINE 105, evaluated the safety and efficacy of the vanza triple in children with CF ages 6 to 11 years with at least one mutation responsive to triple combination CFTRm.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · February 5, 2024
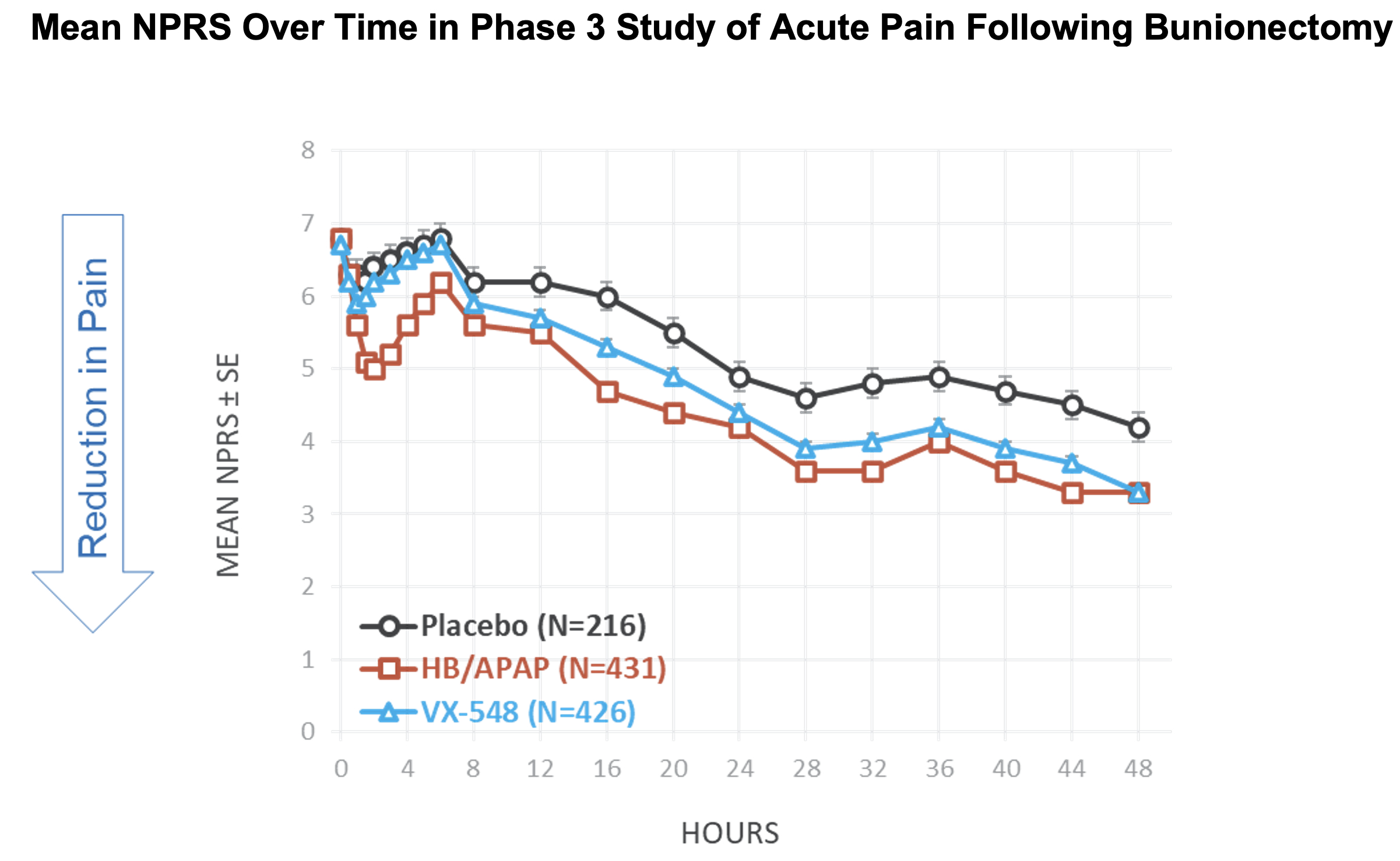
Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced positive results from its Phase 3 program for the selective NaV1.8 inhibitor, VX-548, in the treatment of moderate-to-severe acute pain. The Phase 3 program included two randomized, double-blind, placebo-controlled, pivotal trials, one following abdominoplasty surgery and one following bunionectomy surgery, as well as a single arm safety and effectiveness study which enrolled patients with a broad range of surgical and non-surgical pain conditions.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 30, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) will report its fourth quarter and full year 2023 financial results on Monday, February 5, 2024 after the financial markets close. The company will host a conference call and webcast at 4:30 p.m. ET. To access the call, please dial (833) 630-2124 (U.S.) or +1 (412) 317-0651 (International) and reference the “Vertex Pharmaceuticals Fourth Quarter 2023 Earnings Call.”
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 17, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) announced today that the U.S. Food and Drug Administration (FDA) has approved CASGEVY™ (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 gene-edited cell therapy, for the treatment of transfusion-dependent beta thalassemia (TDT) in patients 12 years and older.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 16, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) announced today that the Saudi Food and Drug Authority (SFDA) granted Marketing Authorization for CASGEVY™ (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 gene-edited therapy, for the treatment of sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT). CASGEVY is approved for the treatment of people 12 years of age and older with SCD or TDT. The Kingdom of Saudi Arabia has among the highest prevalence rates of SCD and TDT in the world, with thousands of patients living with these genetic blood disorders.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 9, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced multiple program updates ahead of upcoming investor meetings in January, including the company’s scheduled webcast from the 42nd Annual J.P. Morgan Healthcare Conference on Monday, January 8, 2024 at 11:15 a.m. ET/8:15 a.m. PT.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · January 7, 2024

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that Dr. Reshma Kewalramani, Chief Executive Officer and President, will present at the 42nd Annual J.P. Morgan Healthcare Conference on Monday, January 8, 2024 at 11:15 a.m. ET/8:15 a.m. PT.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 18, 2023

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) announced today that the European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion for the conditional approval of CASGEVY™ (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 gene-edited therapy, for the treatment of severe sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT).
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 15, 2023

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced positive results from its Phase 2 dose-ranging study of the selective NaV1.8 inhibitor VX-548 in people with painful diabetic peripheral neuropathy (DPN). Treatment with all doses of VX-548 resulted in a statistically significant and clinically meaningful reduction in the primary endpoint of change from baseline in the weekly average of daily pain intensity on a Numeric Pain Rating Scale (NPRS) at Week 12. The study also included an active reference arm of pregabalin to support the evaluation of the VX-548 treatment effect.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 13, 2023

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced two oral presentations at the American Society of Hematology (ASH) Annual Meeting and Exposition from the global pivotal trials of CASGEVY™ (exagamglogene autotemcel [exa-cel]).
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 11, 2023

Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) and CRISPR Therapeutics (Nasdaq: CRSP) announced today that the U.S. Food and Drug Administration (FDA) has approved CASGEVY™ (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 genome-edited cell therapy, for the treatment of sickle cell disease (SCD) in patients 12 years and older with recurrent vaso-occlusive crises (VOCs). This approval means that for the first time, approximately 16,000 patients with SCD may be eligible for a durable one-time therapy that offers the potential of a functional cure for their disease by eliminating severe VOCs and hospitalizations caused by severe VOCs.
By Vertex Pharmaceuticals Incorporated · Via Business Wire · December 8, 2023
