Articles from Stryker

Stryker (NYSE:SYK), a global leader in medical technologies, will showcase new additions to its trauma offerings at the Orthopaedic Trauma Association (OTA) Annual Meeting, October 15–18, in Phoenix, Ariz. (booth #601). The company will highlight upcoming expansions to its nailing and plating platforms.
By Stryker · Via Business Wire · October 14, 2025

Stryker (NYSE:SYK), a global leader in medical technologies, will launch its Incompass™ Total Ankle System at the 2025 American Orthopaedic Foot & Ankle Society (AOFAS) Annual Meeting in Savannah, Georgia, September 10–13, 2025 (booth # 401). The FDA-cleared system is intended for patients with end-stage ankle arthritis and introduces an innovative implant with an enhanced instrument platform.
By Stryker · Via Business Wire · September 9, 2025

Stryker (NYSE:SYK), a global leader in medical technologies, announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Incompass® Total Ankle System, an implant intended for patients with ankle joints damaged by severe rheumatoid, post-traumatic, or degenerative arthritis. This new platform integrates the innovative technologies of Stryker’s Inbone® and Infinity® systems into a single, comprehensive solution for total ankle replacement.
By Stryker · Via Business Wire · June 25, 2025

Stryker (NYSE:SYK), a global leader in medical technologies, announced the addition of two new products to its Foot & Ankle portfolio – the Osteotomy Truss System™ (OTS) and Ankle Truss System™ (ATS), recently acquired from 4WEB Medical. The company will feature both the OTS and ATS, the newly acquired Artelon portfolio, and other products at the American Orthopaedic Foot & Ankle Society (AOFAS) Annual Meeting taking place in Vancouver, Canada from Sept. 11-14, 2024.
By Stryker · Via Business Wire · September 11, 2024
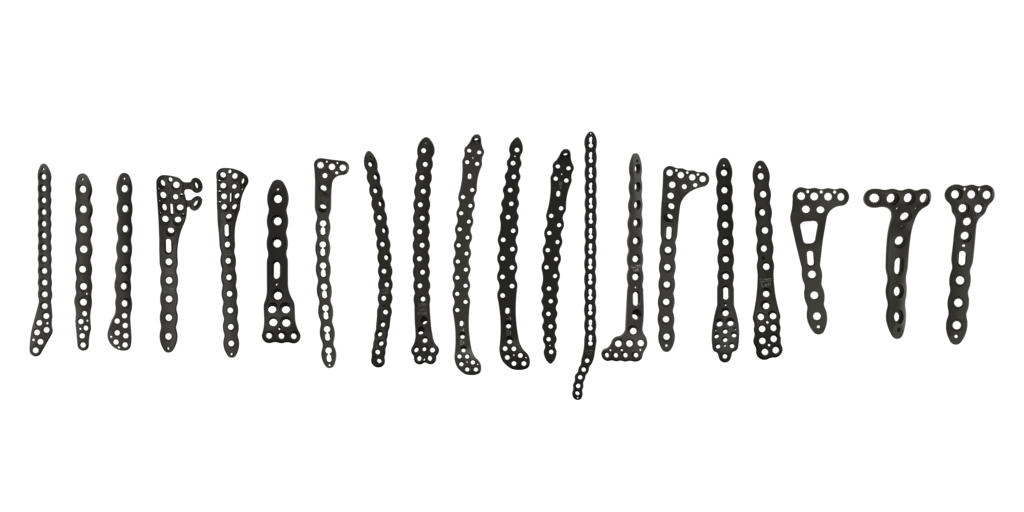
Stryker (NYSE: SYK), a global leader in medical technologies, announced the launch of its Pangea Plating System, which received FDA clearance in late 2023. Pangea offers a comprehensive and versatile portfolio, providing variable-angle plating for a variety of patient populations.
By Stryker · Via Business Wire · August 6, 2024

Stryker (NYSE: SYK), a global leader in medical technologies, has completed the previously announced acquisition of Artelon, a privately held company specializing in innovative soft tissue fixation products for foot and ankle and sports medicine procedures.
By Stryker · Via Business Wire · July 15, 2024

Stryker (NYSE: SYK), a global leader in medical technologies, announced the signing of a definitive agreement to acquire all of the issued and outstanding shares of Artelon, a privately held company specializing in innovative soft tissue fixation products for foot and ankle and sports medicine procedures. The acquisition will strengthen Stryker’s offerings in the soft tissue fixation segment and highlights Stryker’s commitment to providing differentiated solutions for ligament and tendon reconstruction.
By Stryker · Via Business Wire · June 3, 2024

Stryker (NYSE: SYK), a global leader in medical technologies, today announced the launch of Prophecy Footprint, an expansion of the Prophecy Surgical Planning system, offering comprehensive surgical planning across the entire foot. The system will be demonstrated at the American College of Foot and Ankle Surgeons (ACFAS) Annual Meeting, Tampa Bay, Fla., Feb. 1-4 (booth #919).
By Stryker · Via Business Wire · January 31, 2024
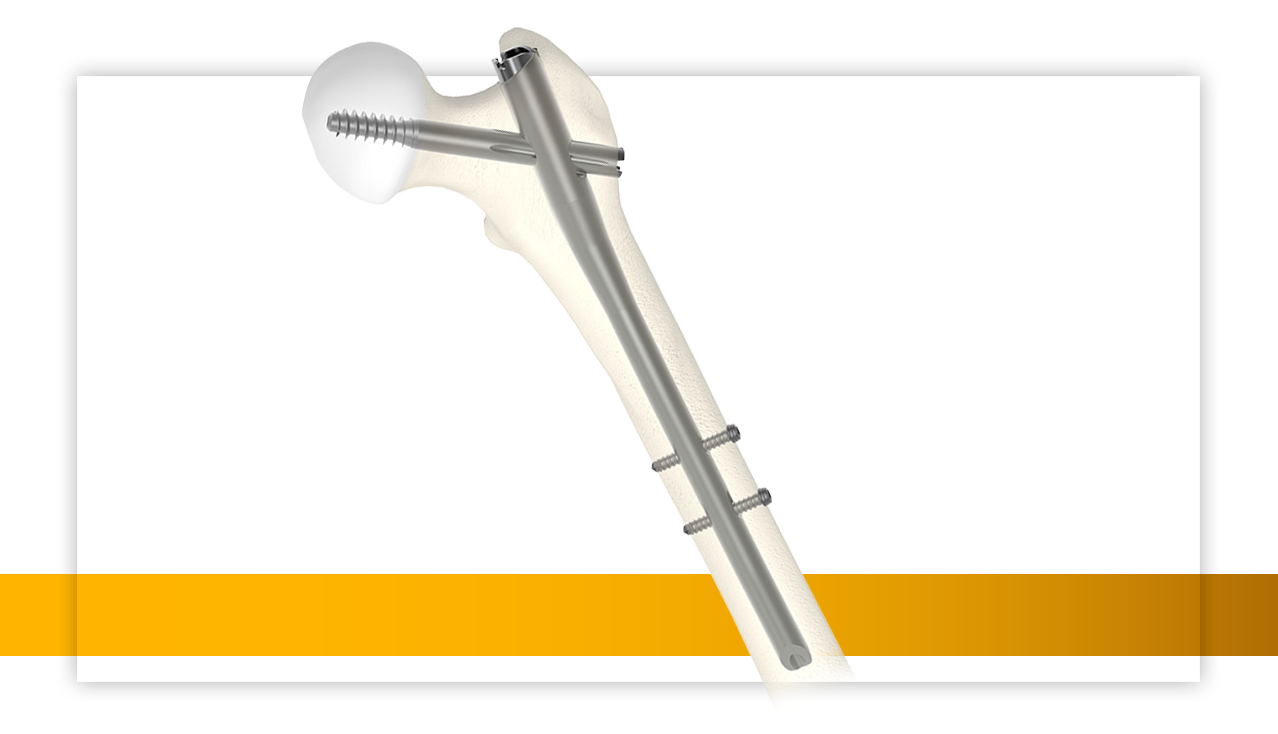
Stryker (NYSE: SYK), one of the world’s leading medical technologies companies, has expanded its Gamma4 Hip Fracture Nailing System, with the addition of an intermediate nail, the RC Lag Screw, and an anti-rotation clip with sleeve components. Since its launch in August of 2022, the Gamma4 System has been used in over 19,000 cases across more than 850 facilities, solidifying its position as a leading choice in orthopaedic procedures.
By Stryker · Via Business Wire · January 17, 2024

Stryker (NYSE:SYK), one of the world’s leading medical technology companies, today announced that the first shoulder arthroplasty surgeries using Blueprint® Mixed Reality (MR) Guidance have been successfully completed by Dr. Joaquin Sanchez-Sotelo, MD, PhD, Consultant and Professor of Orthopedic Surgery at Mayo Clinic in Rochester, Minn., and Dr. George Athwal at St. Joseph’s Health Care London, Canada.
By Stryker · Via Business Wire · January 10, 2024

Stryker (NYSE:SYK), one of the world’s leading medical technology companies, announced today that its Q Guidance System with Spine Guidance Software has been used to deliver enhanced surgical planning and navigation in over 2,400 spinal cases. The Q Guidance System for both spine and cranial applications will be featured at the North American Spine Society (NASS) annual meeting in Los Angeles, Oct. 18 - 21, booth no. 1701.
By Stryker · Via Business Wire · October 12, 2023
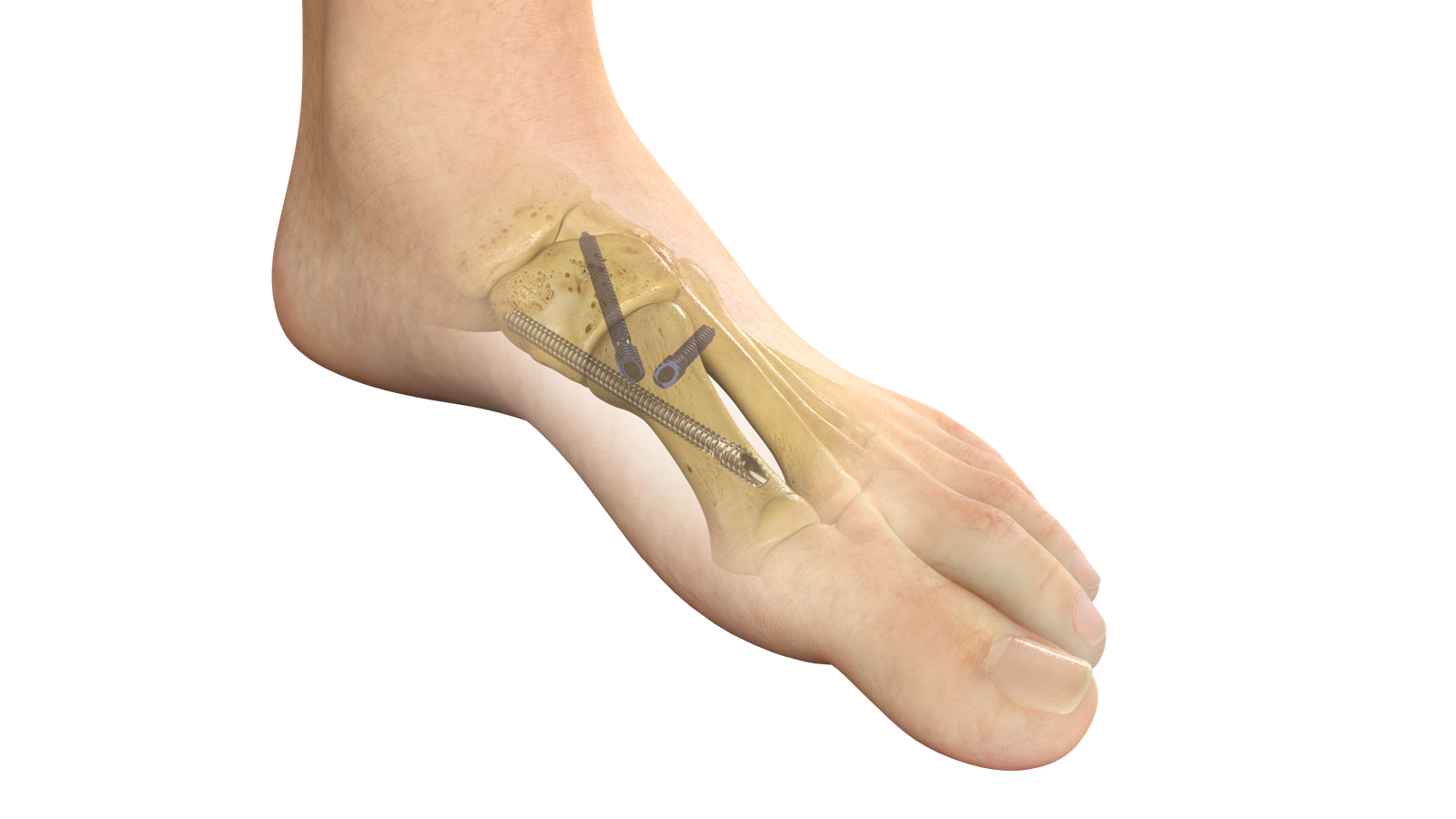
Stryker (NYSE: SYK), one of the world’s leading medical technology companies, announced the launch of PROstep® MIS Lapidus, a new internal fixation system intended for treating bunions using a minimally invasive surgical reduction of hallux valgus deformity and subsequent fusion of the first metatarsal cuneiform joint. The system will debut at the American Orthopaedic Foot & Ankle Society (AOFAS) Annual Meeting in Louisville, Ky., Sept. 20-23 (booth no. 101).
By Stryker · Via Business Wire · September 19, 2023

Stryker (NYSE: SYK), one of the world’s leading medical technology companies, announced that its Pangea Systems including Femur, Fibula, Tibia, Humerus and Utility have received 510k clearance from the U.S. Food & Drug Administration.
By Stryker · Via Business Wire · September 12, 2023

Stryker (NYSE: SYK), one of the world’s leading medical technology companies, has commercially launched its Q Guidance System with Cranial Guidance Software to provide surgeons with an image-based planning and intraoperative guidance system that assists in positioning instruments and identifying patient anatomy during cranial surgery. The software can be used for craniotomies, skull base and transsphenoidal procedures, shunt placements, and biopsies.
By Stryker · Via Business Wire · July 11, 2023
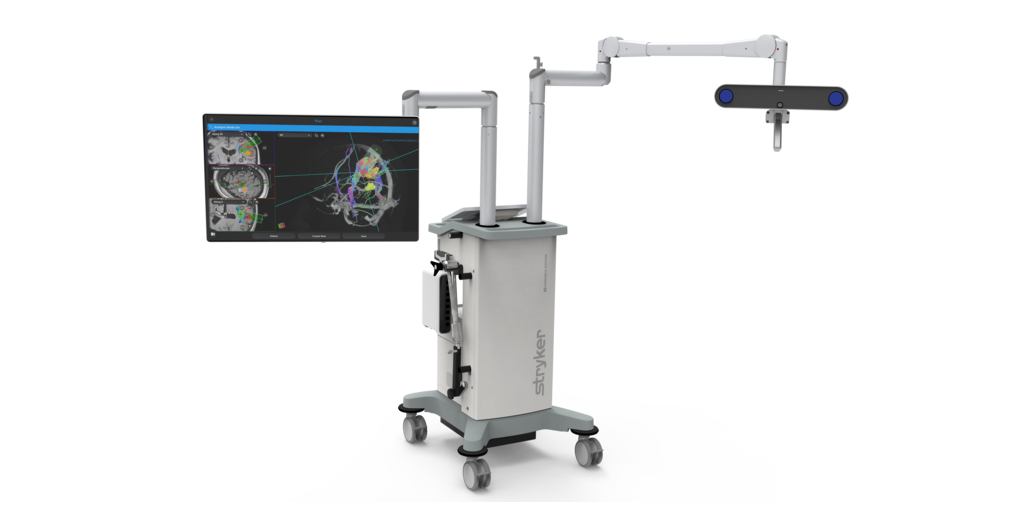
Stryker (NYSE:SYK), one of the world’s leading medical technology companies, announced that Early Product Surveillance (EPS) cases utilizing its Q Guidance System with Cranial Guidance Software are underway. The technology provides surgeons with image-based planning and an intraoperative guidance system designed to support cranial surgeries.
By Stryker · Via Business Wire · May 24, 2023

Stryker (NYSE:SYK), one of the world’s leading medical technology companies, today announced that Armodios M. Hatzidakis, MD, FAAOS, Western Orthopaedics, PC, Denver Surgery Center and Rose Medical Center, has successfully completed the first case in the United States, since the De Novo clearance, using the Tornier Pyrocarbon Humeral Head. Stryker’s De Novo request was granted in late 2022 after completing an Investigational Device Exemption (IDE) study with the FDA.
By Stryker · Via Business Wire · March 30, 2023

Stryker (NYSE:SYK), one of the world’s leading medical technology companies, today announced that its Q Guidance System with Cranial Guidance Software received 510(k) clearance from the U.S. Food and Drug Administration. The Q Guidance System is an image-based planning and intraoperative guidance system designed to support cranial surgeries. Recently launched in September 2022, the Q Guidance System for spinal applications is currently available on the market.
By Stryker · Via Business Wire · February 17, 2023

Stryker (NYSE:SYK) today announced the launch of Citrefix™, a suture anchor system for foot and ankle surgical procedures. The new system uses Citregen™, an award-winning bioresorbable material designed to mimic the chemistry and structure of native bone.1
By Stryker · Via Business Wire · December 13, 2022

Stryker (NYSE:SYK), one of the world’s leading medical technology companies, will feature its suite of procedural solutions for spinal surgery, including its award-winning Q Guidance System with Spine Guidance Software, during the North American Spine Society (NASS) annual meeting in Chicago, Oct. 12–15 (booth #4211).
By Stryker · Via Business Wire · October 12, 2022

Stryker (NYSE:SYK), one of the world’s leading medical technology companies, announced today the launch of the Monterey AL Interbody System, a stand-alone interbody fusion device designed for anterior lumbar interbody fusion (ALIF). Monterey AL is made up of both solid and porous structures within a single implant, leveraging Stryker’s proprietary Tritanium In-Growth Technology, a material designed to mimic cancellous bone and provide an environment favorable to bone regeneration and fusion.1-4 New data demonstrates that undifferentiated stem cells grown on Tritanium exhibited osteogenic Alkaline Phosphatase without requiring growth factor supplements.5,6
By Stryker · Via Business Wire · October 4, 2022
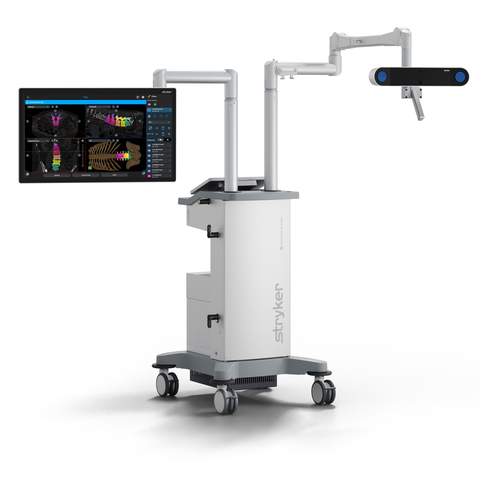
Stryker (NYSE:SYK), one of the world’s leading medical technology companies, announced today the launch of its Q Guidance System for spine applications. The System combines new optical tracking options provided by a redesigned, state-of-the-art camera with sophisticated algorithms of the newly launched Spine Guidance Software to deliver more surgical planning and navigation capability than ever before. When used with the Q Guidance System, the Spine Guidance Software is intended as a planning and intraoperative guidance system to enable open or percutaneous computer-assisted surgery and is the first spine navigation software to receive FDA clearance for use with pediatric patients aged 13 and older.
By Stryker · Via Business Wire · September 27, 2022

Stryker (NYSE:SYK) today announced the launch of its Pulse Intelligent Delivery Platform, a full-circle product and fulfillment offering optimized for high volume foot and ankle procedures in the ambulatory surgery center (ASC) setting, which will debut at the 2022 American Orthopaedic Foot & Ankle Society Annual Meeting (AOFAS), taking place Sept. 14-17 in Québec City, Canada. The company will also feature its Prophecy® Footprint Surgical Planning, which will be the first surgical planning tool to provide clinical guidance on the whole foot in connection with total ankle arthroplasty.
By Stryker · Via Business Wire · September 14, 2022

Stryker (NYSE:SYK) has launched the Gamma4 System, strengthening the product’s 30-year legacy of continuous innovation and clinical history. The newest Gamma System will provide surgeons with the next generation of Stryker’s intramedullary nailing system.
By Stryker · Via Business Wire · September 1, 2022

Stryker (NYSE:SYK), one of the world’s leading medical technology companies, today launched an experiential campaign to inspire consumers to explore new treatment options for bunions and increase awareness of the benefits of bunion surgery. The interactive campaign consists of visually creative chalk designs in four major metropolitan cities – New York City, Los Angeles, Chicago and Miami – prompting passersby to learn more.
By Stryker · Via Business Wire · June 28, 2022

Stryker (NYSE:SYK) today announced that its Q Guidance System received 510(k) clearance from the U.S. Food and Drug Administration. The System, when used with the Spine Guidance Software, is an advanced planning and intraoperative guidance system designed to enable open or percutaneous computer-assisted surgery. The Spine Guidance Software is the first spine navigation software to receive clearance from the FDA for use with pediatric patients aged 13 and older.
By Stryker · Via Business Wire · May 31, 2022

Stryker (NYSE:SYK) today announced the launch of its EasyFuse™ Dynamic Compression System. Created using nitinol, a nickel titanium alloy metal well known for its strength and shape recovery, Stryker’s new foot and ankle staple system is designed to decrease surgical complexity, provide strong dynamic-compression implants and reduce waste in the operating room.
By Stryker · Via Business Wire · May 25, 2022

Stryker, one of the world’s leading medical technology companies, will debut the new PROstep™ MICA® SOLO Guide at the American College of Foot and Ankle Surgery (ACFAS) 2022 meeting and will host a live event on March 2 at 7 p.m. EST. The all-in-one procedure guide is used during PROstep MICA minimally invasive bunion procedures and is designed for use by a solo surgeon, potentially eliminating the need for additional surgical assistance during the operation.
By Stryker · Via Business Wire · February 24, 2022

Stryker, one of the world’s leading medical technology companies, announced today that its new Tornier Perform® Patient-Matched Primary Reversed Glenoid (Patient-Matched Glenoid) was used in a surgical procedure for the first time. The case was completed Jan. 13 by Jay Keener, M.D., in St. Louis and will be highlighted in a presentation at the 2022 Advanced Shoulder Arthroplasty (ASAP) Meeting on Jan. 21 in Snowbird, Utah.
By Stryker · Via Business Wire · January 20, 2022

Stryker today launched the Prophecy® Infinity® Resect-Through Guides for use in total ankle replacement surgeries. The system represents the latest refinement of Stryker’s current industry gold standard technique, allowing surgeons to improve OR efficiency by reducing potentially time-consuming interoperative steps in a streamlined surgical process.
By Stryker · Via Business Wire · November 16, 2021
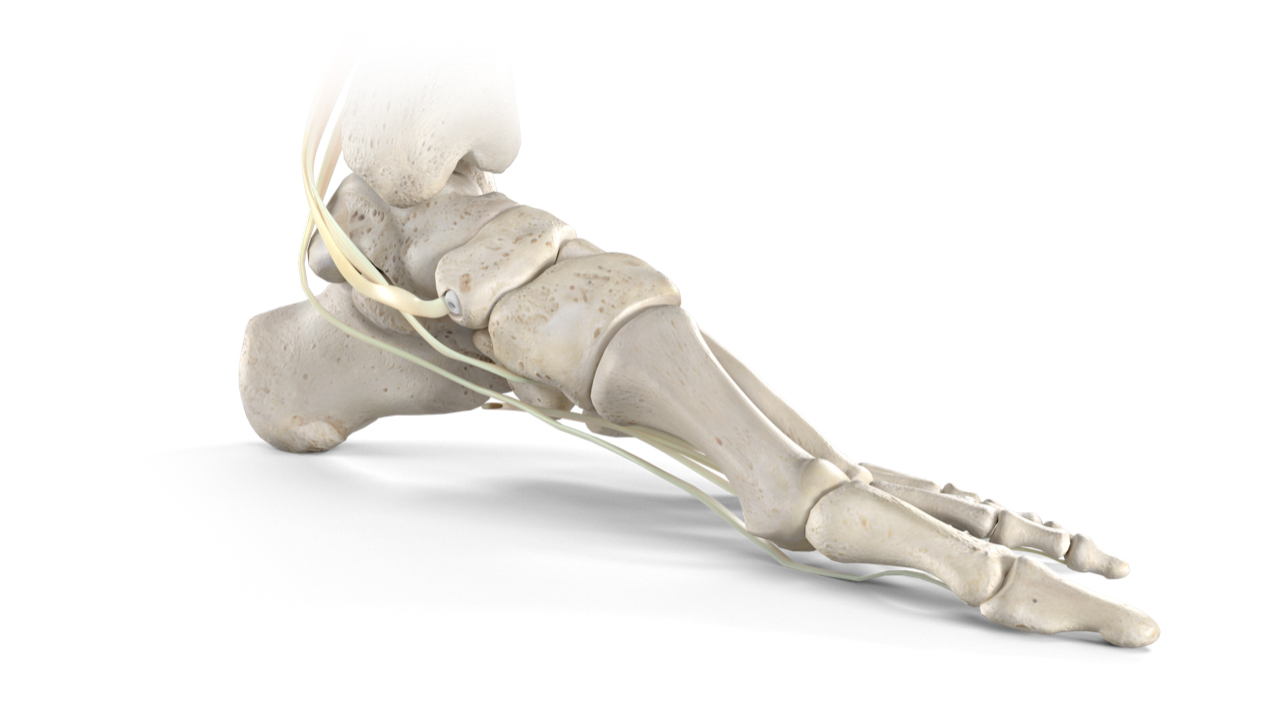
Stryker’s Trauma & Extremities division has launched its Citrelock™ Tendon Fixation Device System. The new system provides surgeons a differentiated design via a tendon thread featuring a resorbable technology, known as Citregen™, that has unique chemical and mechanical properties for orthopaedic surgical applications. Stryker will debut Citrelock at the American Orthopaedic Foot & Ankle Society (AOFAS) Annual Meeting, Sept. 22-25, 2021, in Charlotte, N.C.
By Stryker · Via Business Wire · September 15, 2021

Stryker has announced the launch of its T2 Alpha Femur Retrograde Nailing System. The new nailing solution features a redefined data-driven nail design,1,2 an advanced locking screw option that provides surgeons with axial stability where desired, and a dedicated proximal targeting system, which may reduce the number of X-ray shots compared to freehand locking.4-7
By Stryker · Via Business Wire · August 24, 2021

Stryker, one of the world’s leading medical technology companies, announced today that it will be a top 20 sponsor of the 2021 World Golf Championships-FedEx St. Jude Invitational. The PGA TOUR event will be held Aug. 2-8, 2021, at TPC Southwind in Memphis, Tenn.
By Stryker · Via Business Wire · July 30, 2021

Stryker, one of the world’s leading medical technology companies, has officially introduced the Tornier shoulder arthroplasty portfolio and launched its first new Tornier product, the Perform™ Humeral Stem.
By Stryker · Via Business Wire · July 13, 2021

Stryker, one of the world’s leading medical technology companies, announced today that it will showcase its new, combined foot and ankle portfolio incorporating Wright Medical’s products at the 2021 American College of Foot and Ankle Surgeons (ACFAS) Vegas Scientific Conference from May 18-21, 2021.
By Stryker · Via Business Wire · May 18, 2021

Today, Stryker and Minor League Baseball™ (MiLB™) announced a first-of-its-kind multiyear national partnership, designating the medical device company as “The Official SmartRobotics™ Joint Replacement Partner of Minor League Baseball.” In support of its commitment to making healthcare better, and as part of the broader MiLB partnership, Stryker is also the presenting partner of “Own the Walk” – the first-ever national platform that encourages fans of all ages to stay active through everyday activities like walking.
By Stryker · Via Business Wire · April 29, 2021
