Articles from Penumbra, Inc.

Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced the first full body, non-tethered immersive healthcare offering for rehabilitation. The latest REAL y-Series product uses upper and lower body sensors that allow clinicians to track full body movement and progress in real time. The expansion of the virtual reality-based immersive healthcare platform, REAL System, will be used to support a broad range of physical, cognitive and mental well-being for patients undergoing physical or occupational therapy.
By Penumbra, Inc. · Via Business Wire · November 15, 2022
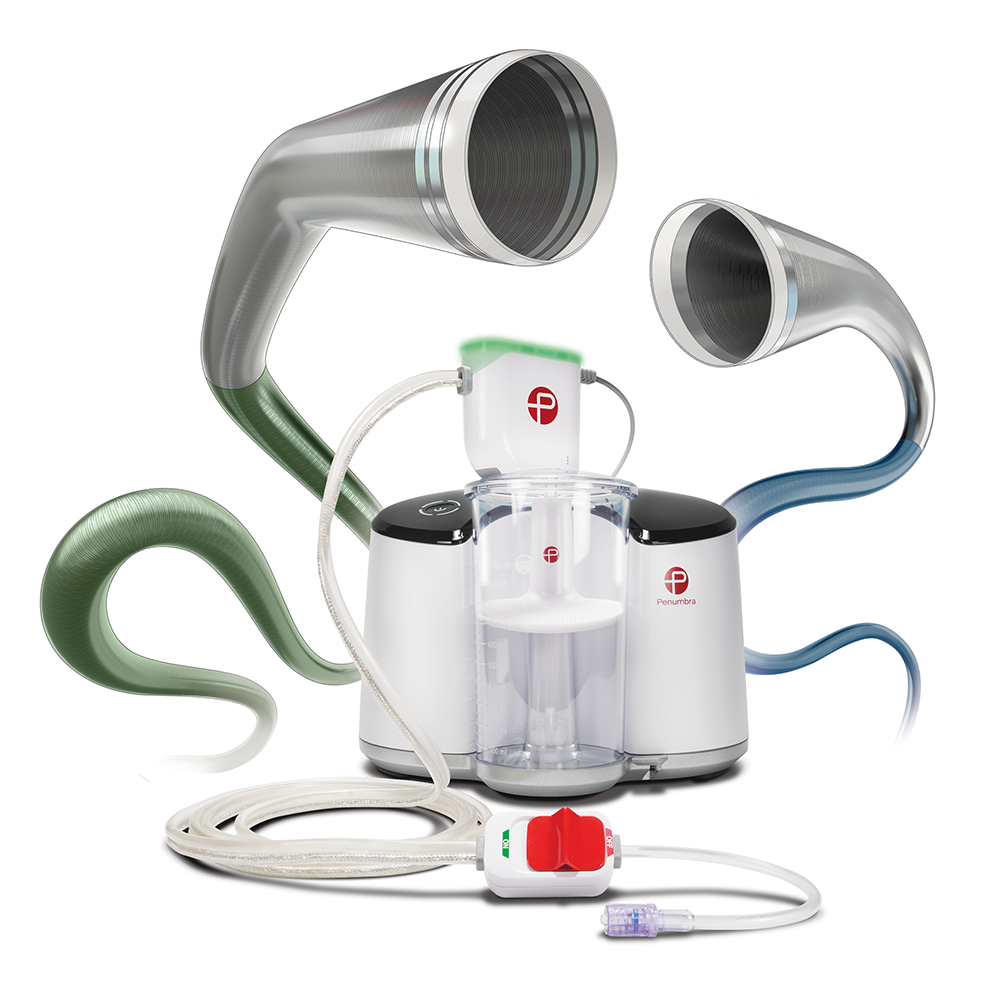
Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, and Asahi Intecc Co., a leading Japanese medical device manufacturer, announced that they will collaborate to introduce Penumbra’s Indigo™ Aspiration System into the Japanese market upon regulatory approval.
By Penumbra, Inc. · Via Business Wire · September 22, 2022

Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, announced that its RED® Reperfusion Catheters have secured CE Mark (Conformité Européenne) and are now commercially available in Europe. The catheters are part of the company’s Penumbra System®, which is a fully integrated mechanical aspiration thrombectomy system designed to restore blood flow in acute ischemic stroke (AIS) patients.
By Penumbra, Inc. · Via Business Wire · September 7, 2022

Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, announced initial INSIGHT Registry data showing Penumbra’s RED® reperfusion catheters were successful in removing all clot types. Additionally, results of sub-analyses of the COMPLETE study showed use of the Penumbra System is effective for acute ischemic stroke (AIS) patients with tandem lesions, as well as patients who are considered in the late window of treatment. The results were presented at the 2022 World Federation of Interventional and Therapeutic Neuroradiology (WFITN) in Kyoto, Japan.
By Penumbra, Inc. · Via Business Wire · August 25, 2022

Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, announced that its Indigo Aspiration System with Lightning 7 and Lightning 12 have secured CE Mark (Conformité Européenne) and are now commercially available in Europe. Both technologies are part of Penumbra’s Indigo Aspiration System – now with Intelligent Aspiration for mechanical thrombectomy – and are designed for single session arterial and venous thrombus removal, including the treatment of pulmonary embolisms.
By Penumbra, Inc. · Via Business Wire · April 14, 2022
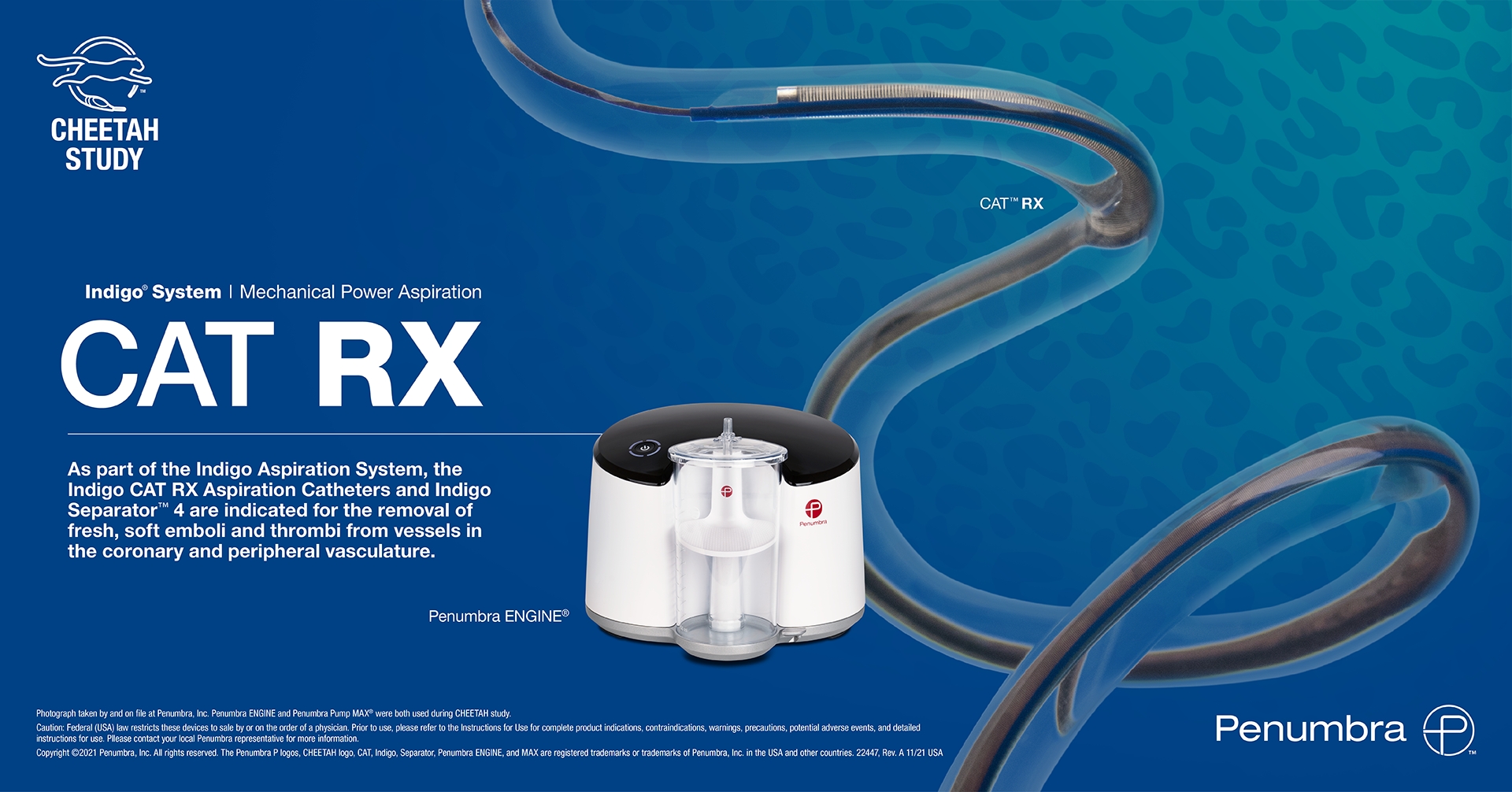
Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, announced that the CHEETAH clinical study of its Indigo® System CAT™ RX Catheter successfully met the primary endpoint and demonstrated high rates of blood clot removal, blood flow restoration and myocardial perfusion in conjunction with percutaneous coronary intervention (PCI) in patients with high thrombus burden. The results were presented at the 2021 Transcatheter Cardiovascular Therapeutics (TCT) conference in Orlando.
By Penumbra, Inc. · Via Business Wire · November 5, 2021

Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced the introduction of the REAL® Immersive System i-Series™, offering engaging, immersive, gaze-based experiences and activities to care providers and mental health professionals. The REAL i-Series features a virtual reality-enabled headset with intuitive gaze navigation and exclusive experiences and activities, including REAL Connect™, a first-to-market advanced capability, providing one-to-one real-time video and audio communication.
By Penumbra, Inc. · Via Business Wire · August 10, 2021

Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance and commercial availability of the RED™ 62 Reperfusion Catheter, the latest addition to the company’s comprehensive Penumbra System®. RED 62 is designed to navigate complex distal vessel anatomy and deliver powerful aspiration, together with Penumbra ENGINE®, for the removal of blood clots in acute ischemic stroke patients with large vessel occlusions.
By Penumbra, Inc. · Via Business Wire · June 23, 2021
