Articles from Paragon 28, Inc.

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, is pleased to announce its participation in the American College of Foot and Ankle Surgeons (ACFAS) Annual Scientific Conference. From March 27-29, Paragon 28 will feature a suite of recently launched products designed to improve foot and ankle patient outcomes and surgeon experience.
By Paragon 28, Inc. · Via Business Wire · March 24, 2025

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or “Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (the “HSR Act”) in connection with the previously announced acquisition of Paragon 28 by Zimmer Biomet Holdings, Inc. (NYSE: ZBH) (“Zimmer Biomet”).
By Paragon 28, Inc. · Via Business Wire · March 11, 2025

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, is pleased to announce its participation in the American Academy of Orthopaedic Surgeons (“AAOS”).
By Paragon 28, Inc. · Via Business Wire · March 7, 2025

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or “P28”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced a range of its preliminary unaudited net revenue for the fourth quarter and full year ended December 31, 2024.
By Paragon 28, Inc. · Via Business Wire · January 13, 2025

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO and Chadi Chahine, CFO & EVP of Supply Chain Operations, will present at the 43rd Annual J.P. Morgan Healthcare Conference on Monday, January 13, 2025 at 7:30 a.m. Pacific Time / 8:30 a.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · January 3, 2025

Paragon 28, Inc. (NYSE: FNA), (the “Company”) a leader in foot and ankle surgical solutions, today announced the appointment of Dave Demski to serve as an independent director of the Company, effective immediately. Mr. Demski brings a wealth of global orthopedic expertise, with over two decades of executive leadership experience. With the addition of Mr. Demski, the Company increased the size of its Board of Directors from eight members to nine members and has further strengthened its strategic oversight and governance capabilities.
By Paragon 28, Inc. · Via Business Wire · December 11, 2024

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO, and Chadi Chahine, CFO, will participate in a fireside chat at the 36th Annual Piper Sandler Healthcare Conference on Tuesday, December 3, 2024, at 8:30 a.m. Eastern Time / 6:30 a.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · November 18, 2024

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or "Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended September 30, 2024 and raised its 2024 net revenue guidance.
By Paragon 28, Inc. · Via Business Wire · November 12, 2024

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the third quarter 2024 after market close on Tuesday, November 12, 2024. The Company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time / 2:30 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · October 17, 2024
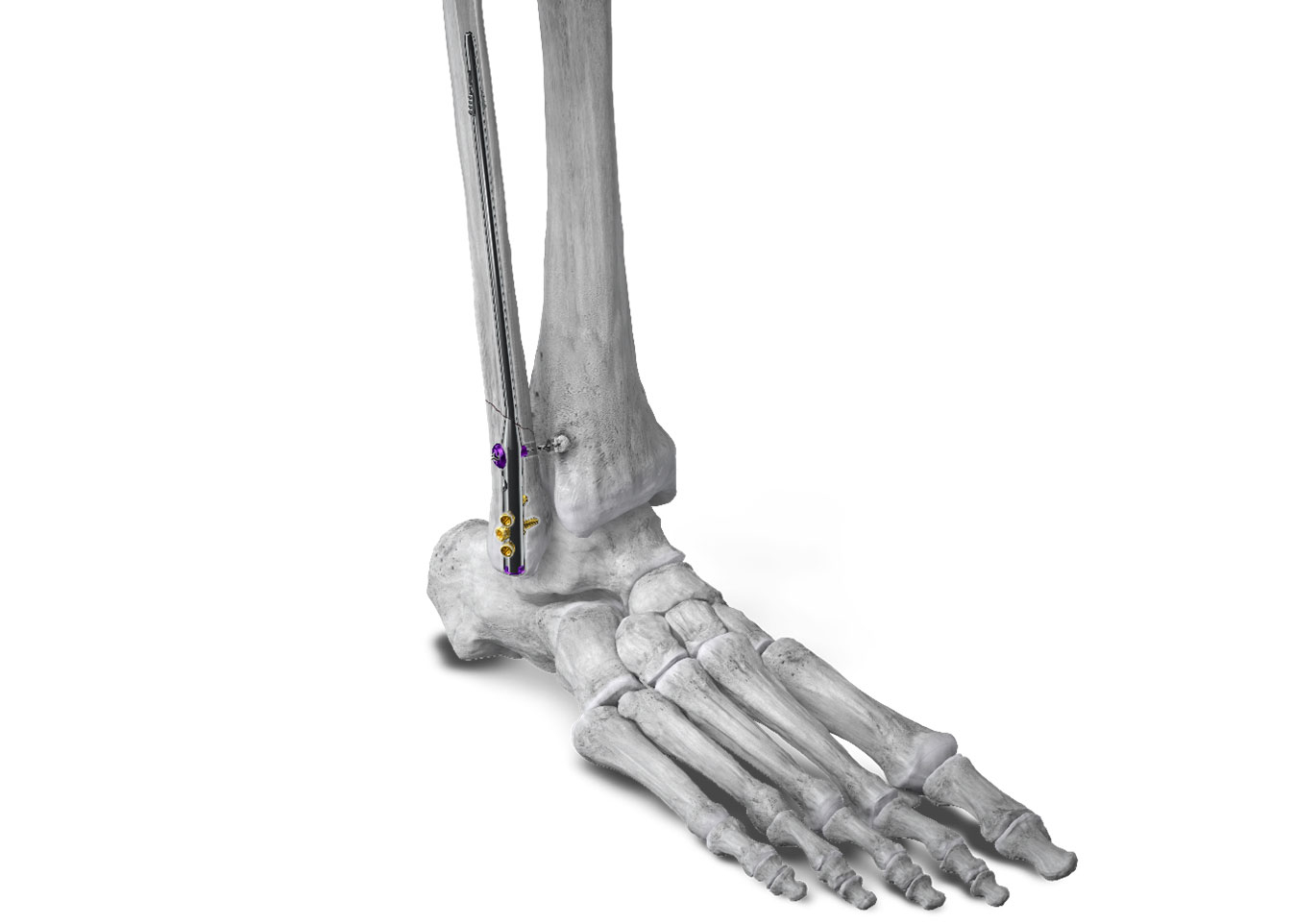
Paragon 28, Inc. (NYSE: FNA) is pleased to announce the launch of the Phantom® Fibula Nail System, designed to give surgeons a less invasive option to treat the fibula when patients sustain an ankle fracture. Use of a fibula nail for the treatment of ankle fractures has been shown to have significantly fewer soft tissue complications, implant removals, and fracture nonunion when compared to plate and screw fixation, a common alternative approach.1
By Paragon 28, Inc. · Via Business Wire · October 16, 2024
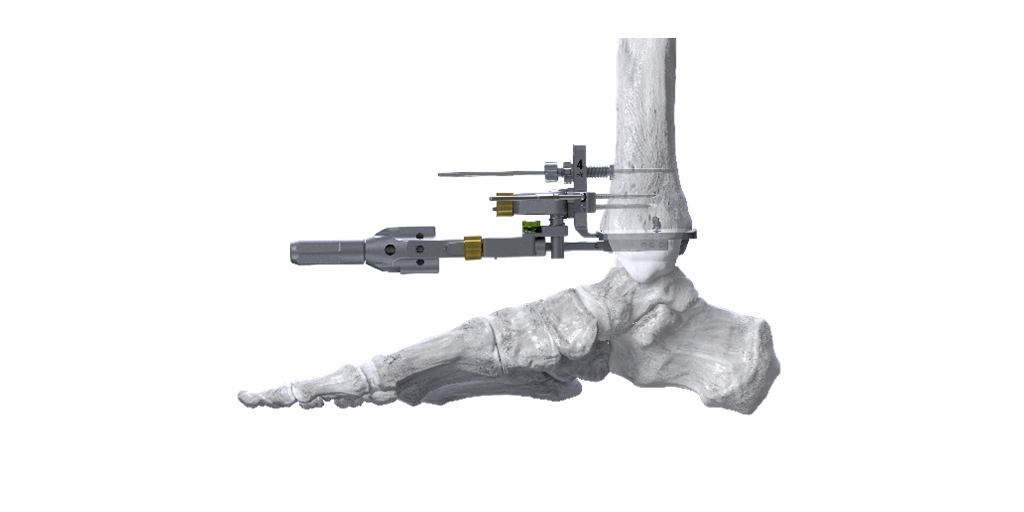
Paragon 28, Inc. (NYSE: FNA) is pleased to announce the addition of a novel Right-Angle Drill to the APEX 3D™ Total Ankle Replacement System designed to improve tibia preparation prior to the implantation of the APEX 3D™ tibia implant. The Right-Angle Drill utilizes a linear guide to precisely drill vertical holes into the tibia for ideal peg placement and a highly stable tibia implant interface.
By Paragon 28, Inc. · Via Business Wire · September 10, 2024
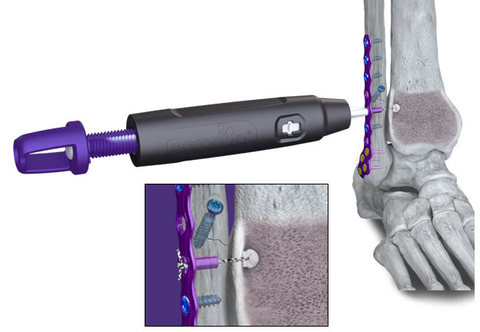
Paragon 28, Inc. (NYSE: FNA), is pleased to announce the launch of the R3FLEX™ Stabilization System, which is designed to restore stability to the ankle syndesmosis after injury from an ankle fracture or high ankle sprain.
By Paragon 28, Inc. · Via Business Wire · September 4, 2024
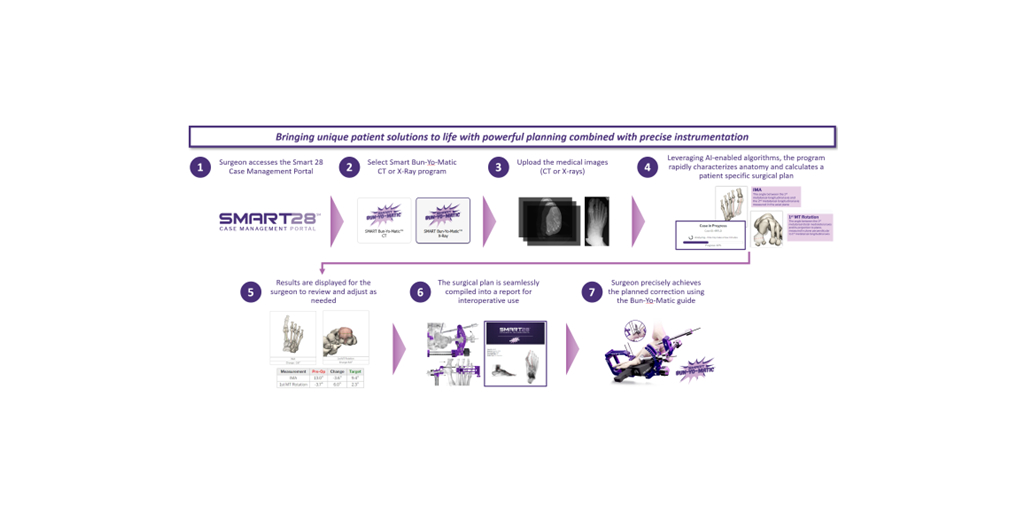
Paragon 28, Inc. (NYSE: FNA), is proud to announce the launch of the SMART28℠ Case Management Portal, a cutting-edge platform that leverages AI and a seamless user experience to coordinate patient-specific surgical plans. It is the first major launch of Paragon 28’s SMART28℠ ecosystem, a portfolio of solutions and products that drive Paragon 28’s SMART28℠ initiative: to improve all aspects of foot and ankle treatments through the utilization of artificial intelligence, data analytics, patient specific algorithms, 3D modeling, and other enabling technologies.
By Paragon 28, Inc. · Via Business Wire · August 14, 2024

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or "Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Chadi Chahine has been appointed Chief Financial Officer and Executive Vice-President of Supply Chain Operations, effective August 5, 2024. The Company also reported financial results for the quarter ended June 30, 2024, and narrowed its 2024 net revenue guidance.
By Paragon 28, Inc. · Via Business Wire · August 8, 2024

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the second quarter 2024 after market close on Thursday, August 8, 2024. The Company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time / 2:30 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · July 17, 2024

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or "Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended March 31, 2024, and reaffirmed its 2024 net revenue guidance.
By Paragon 28, Inc. · Via Business Wire · May 8, 2024

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO, and Krissy Wright, Interim CFO, will participate in a fireside chat at the Bank of America Securities 2024 Healthcare Conference on Tuesday, May 14, 2024, at 5:00 p.m. Pacific Time / 6:00 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · May 2, 2024

Paragon 28, Inc. (NYSE: FNA), (“Paragon 28” or “the Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Stephen Deitsch, Chief Financial Officer (“CFO”), has notified the Company of his intent to pursue an opportunity with OrganOx Limited, a commercial stage UK-based medical device company focused on therapeutic applications of isolated organ perfusion. Mr. Deitsch will depart from the Company on April 5, 2024. The Company has formed a sub-committee of its Board of Directors and has engaged an executive search firm to assist in identifying a permanent successor.
By Paragon 28, Inc. · Via Business Wire · April 4, 2024

Paragon 28, Inc. (NYSE: FNA), is pleased to announce the launch of the Grappler® R3INFORCE™ Extraosseous Repair System designed to restore stability to the anterior and posterior ligaments surrounding the ankle during fibula fracture repairs and high ankle sprains.
By Paragon 28, Inc. · Via Business Wire · March 28, 2024

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or “Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter and year ended December 31, 2023 and provided 2024 Net Revenue guidance.
By Paragon 28, Inc. · Via Business Wire · February 29, 2024

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO and Steve Deitsch, CFO will participate in a fireside chat at the CG 2024 Musculoskeletal Conference on Monday, February 12, 2024, at 1:00 p.m. Pacific Time / 2:00 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · February 12, 2024

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the fourth quarter and full year 2023 after market close on Thursday, February 29, 2024. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time / 2:30 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · February 6, 2024
Paragon 28, Inc. (NYSE: FNA) is pleased to announce the launch of the PRECISION® MIS Bunion System, which allows surgeons to complete a distal metatarsal osteotomy using a minimally invasive (MIS) surgical technique.
By Paragon 28, Inc. · Via Business Wire · February 1, 2024

Paragon 28, Inc. (NYSE: FNA) is proud to announce a novel solution in modernizing foot and ankle surgery with the launch of its FJ2000™ Power Console and Burr System designed for a wide range of minimally invasive and open procedures in the foot and ankle.
By Paragon 28, Inc. · Via Business Wire · January 30, 2024

Paragon 28, Inc. (NYSE: FNA), is pleased to announce the launch of the Mister Tendon™ System which allows surgeons to perform a distal cut of the flexor hallucis longus (“FHL”) or flexor digitorum longus (“FDL”) tendon through a minimally invasive incision, harvesting a working length of tendon suitable for tendon transfer procedures. Use of this instrumentation through a minimally invasive approach, is designed to allow for better healing and quicker recovery time in flatfoot reconstruction. In addition to the harvester, an accessory dilator instrument is provided to help bluntly dissect soft tissue, improving harvester access without causing additional damage.
By Paragon 28, Inc. · Via Business Wire · January 26, 2024

Paragon 28, Inc. (NYSE: FNA), is excited to announce the limited market release of its Bun-Yo-Matic™ Lapidus Clamp System and completion of the first surgical cases in Orange County, California and Seattle, Washington. The Bun-Yo-Matic™ is the only bunion solution on the market designed to intraoperatively simulate weight-bearing and allow for compression prior to hardware application. This unique approach provides surgeons a more reproducible and efficient means to restore three-dimensional alignment of the foot while accommodating each surgeon’s preferred fixation methodology in a Lapidus procedure.
By Paragon 28, Inc. · Via Business Wire · January 17, 2024

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or “P28”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced a range of its preliminary unaudited net revenue for the fourth quarter and full year ended December 31, 2023.
By Paragon 28, Inc. · Via Business Wire · January 8, 2024
Paragon 28, Inc. (NYSE: FNA), is thrilled to announce the launch of the Grappler® Knotless Anchor System and Bridgeline™ Tape, enhancing the Company’s position in the fast-growing foot and ankle specific soft tissue market in 2024 and beyond. Both the Grappler® Knotless Anchor System and Bridgeline™ Tape are highly complementary to a broad range of Paragon 28’s existing hardware products with several applications spanning multiple foot and ankle indications.
By Paragon 28, Inc. · Via Business Wire · January 5, 2024

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO and Steve Deitsch, CFO will present at the 42nd Annual J.P. Morgan Healthcare Conference on Monday, January 8, 2024 at 3:00 p.m. Pacific Time / 4:00 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · December 19, 2023

Paragon 28, Inc. (NYSE: FNA) is pleased to announce the launch of its BEAST™ Cortical Fibers which provide an osteoinductive porous structure for cellular attachment and an osteoinductive potential to aid in cellular differentiation and bone formation. These loose cortical fibers are engineered to complement specific surgical applications in the foot and ankle and are flexible upon hydration allowing for application even in extremely challenging fusion locations. The cortical matrix absorbs bioactive fluid including bone marrow aspirate and supports cellular infiltration to drive efficient bone remodeling. Powerful processing capability allows for preservation of native bone morphologic proteins (BMPs) and other growth factors necessary for the promotion of bone formation.
By Paragon 28, Inc. · Via Business Wire · November 14, 2023
Paragon 28, Inc. (NYSE: FNA) is pleased to announce the launch of its JAWS™ Great White Staple System which was developed to provide for increased strength and stability of the osteotomy or fusion site when compared to traditional staple systems. The staples feature an ultra-low-profile bridge with increased surface area to enhance stability while minimizing soft tissue irritation. The newly designed staple has 400 times the fatigue life compared to a competitive nitinol staple subjected to the same load parameters.1 The shoulders of the staple work in conjunction with the inserter allowing the staple to be fully seated prior to compression activation limiting the need to tamp. The staples were designed to provide a uniform compression profile across the osteotomy and provide a 169% increased compressive force when compared to the average of a competitive marketing leading two-prong, nitinol staple.1
By Paragon 28, Inc. · Via Business Wire · November 14, 2023

Paragon 28, Inc. (NYSE: FNA) is pleased to announce the addition of a second trailer to its nationwide mobile lab training program making it even more convenient for the company to facilitate on-site surgeon training and education. Paragon 28’s new mobile lab is housed in a 40 foot tractor-trailer which includes a state-of-the-art, 5 station, cadaveric training facility accommodating up to 25 surgeons. The mobile lab will host over 70 training sessions in approximately 65 US cities during quarter four of 2023. The mobile lab program kicked off in July of 2022 and has been an incredible success for Paragon 28, allowing the company to efficiently train over 350 surgeons.
By Paragon 28, Inc. · Via Business Wire · November 13, 2023

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or "Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended September 30, 2023 and reaffirmed its 2023 net revenue guidance.
By Paragon 28, Inc. · Via Business Wire · November 7, 2023

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or "Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that it has received a new $150 million credit facility from Ares Capital Corporation (“the Facility”) to replace its existing $90 million senior credit facility. The Facility is comprised of up to $100 million in term loans, with $75 million drawn at close, and a $50 million revolving credit facility, with $25 million drawn at close. The Facility is non-dilutive without warrants or other equity-based instruments. The Company’s September 30, 2023, pro forma liquidity is $147.0 million, including $97.0 million of pro forma cash and $50 million of available borrowings under the Facility.
By Paragon 28, Inc. · Via Business Wire · November 7, 2023

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO and Steve Deitsch, CFO will be presenting in fireside chats at two upcoming investor conferences. Paragon 28 will participate in the Stephens Annual Investment Conference on November 15, 2023, and the 35th Annual Piper Sandler Healthcare Conference on November 28, 2023.
By Paragon 28, Inc. · Via Business Wire · November 3, 2023

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the third quarter 2023 after market close on Tuesday, November 7, 2023. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time / 2:30 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · October 17, 2023
Paragon 28, Inc. (NYSE: FNA), announced today they have received an Investigational Device Exemption (IDE) approval from the FDA to commence a feasibility study for configurations of the SMART Total Talus™ System used in conjunction with the Paragon 28® APEX 3D™ Total Ankle Replacement System. The study is expected to begin in early 2024.
By Paragon 28, Inc. · Via Business Wire · September 11, 2023

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO and Steve Deitsch, CFO will participate in a fireside chat at the Morgan Stanley 21st Annual Global Healthcare Conference on Monday, September 11, 2023, at 4:55 p.m. Eastern Time / 2:55 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · August 28, 2023

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or "Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended June 30, 2023 and reaffirmed its 2023 net revenue guidance.
By Paragon 28, Inc. · Via Business Wire · August 2, 2023

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO, and Steve Deitsch, CFO, will participate in a fireside chat at the Canaccord Genuity 43rd Annual Growth Conference on Wednesday, August 9, 2023, at 8:30 a.m. Eastern Time / 6:30 a.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · July 28, 2023

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the second quarter 2023 after market close on Wednesday, August 2, 2023. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time / 2:30 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · July 12, 2023
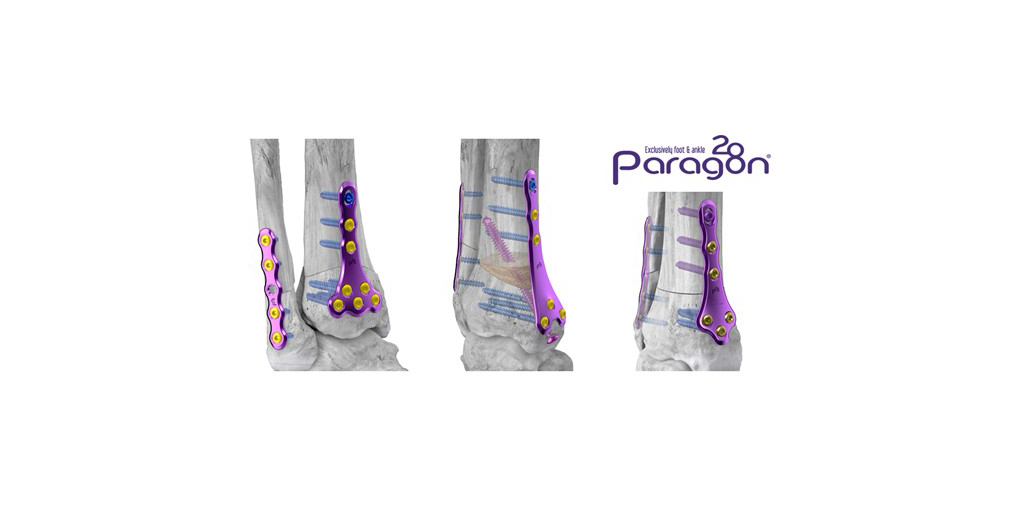
Paragon 28, Inc. (NYSE: FNA) is pleased to announce the launch of its Gorilla® Supramalleolar Osteotomy (SMO) Plating and PRESERVE™ SMO Allograft System which were developed to provide surgeons versatility in plate selection and surgical approach for supramalleolar osteotomies. The system includes patent pending drilling and cutting guides to facilitate repeatable and controlled anterior dome, medial opening, and closing wedge osteotomies. The system also includes a patent pending allograft cutting jig designed to shape the PRESERVE™ SMO Allograft Wedge to match the intended correction while reducing time and technicality of the procedure. Six plates are included in the system for anterior or medial approaches and include multiple spanning distances to accommodate varying correction.
By Paragon 28, Inc. · Via Business Wire · May 23, 2023

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or "Company”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended March 31, 2023 and reaffirmed its 2023 net revenue guidance.
By Paragon 28, Inc. · Via Business Wire · May 4, 2023

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO, and Steve Deitsch, CFO, will participate in a fireside chat at the Bank of America Securities 2023 Healthcare Conference on Wednesday, May 10, 2023, at 5:20 p.m. Eastern Time / 3:20 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · April 26, 2023

Paragon 28, Inc. (NYSE: FNA), is pleased to announce the launch of its Phantom® Metatarsal Shortening System which features a first in-kind intramedullary device for fixation of in-line shortening osteotomies of the lesser metatarsals. These osteotomies are preformed to prevent metatarsalgia and floating toe, two of the most frequently addressed foot and ankle pathologies. The system includes a patent pending cut guide allowing for increased precision when performing the correction.
By Paragon 28, Inc. · Via Business Wire · April 24, 2023

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the first quarter 2023 after market close on Thursday, May 4, 2023. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time / 2:30 p.m. Mountain Time.
By Paragon 28, Inc. · Via Business Wire · April 20, 2023

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter and year ended December 31, 2022.
By Paragon 28, Inc. · Via Business Wire · March 2, 2023

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO, and Steve Deitsch, CFO, will be presenting in fireside chats at two upcoming investor conferences. Paragon 28 will participate in the CG 2023 Musculoskeletal Conference on March 7th, 2023, and the 22nd Annual Needham Virtual Healthcare Conference on April 19th, 2023.
By Paragon 28, Inc. · Via Business Wire · March 1, 2023

Paragon 28, Inc. (NYSE: FNA) (“Paragon”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today completion of the sale of an additional 975,000 shares of its common stock, pursuant to the exercise in full of the option to purchase additional shares granted to the underwriters in connection with Paragon’s recently completed underwritten public offering of 6,500,000 shares of common stock, at a public offering price of $17.00 per share, before underwriting discounts and commissions. 562,500 shares were sold by Paragon and 412,500 shares were sold by certain selling securityholders. After giving effect to the sale of these additional shares, a total of 4,312,500 shares were sold by Paragon and 3,162,500 shares were sold by the selling securityholders in the offering. Paragon received aggregate gross proceeds of approximately $73.3 million in the offering, before deducting underwriting discounts and commissions and other estimated offering expenses payable by Paragon. Paragon did not receive any proceeds from the sale of common stock by the selling securityholders.
By Paragon 28, Inc. · Via Business Wire · February 17, 2023

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the fourth quarter and full year 2022 after market close on Thursday, March 2, 2023. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time.
By Paragon 28, Inc. · Via Business Wire · February 16, 2023

Paragon 28, Inc. (NYSE: FNA) (“Paragon”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today the pricing of the previously announced underwritten public offering of 6,500,000 shares of its common stock at a public offering price of $17.00 per share, before underwriting discounts and commissions. 3,750,000 shares in the offering are being offered for sale by Paragon and 2,750,000 shares are being offered for sale by certain selling securityholders. In addition, Paragon and the selling securityholders have granted the underwriters a 30-day option to purchase up to an additional 562,500 and 412,500 shares of Paragon’s common stock, respectively, at the public offering price, less underwriting discounts and commissions. The gross proceeds from the offering to Paragon are expected to be approximately $63.8 million. Paragon will not receive any proceeds from the sale of common stock by the selling securityholders. The offering is expected to close on January 30, 2023, subject to customary closing conditions.
By Paragon 28, Inc. · Via Business Wire · January 25, 2023

Paragon 28, Inc. (NYSE: FNA) (“Paragon”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today the commencement of a proposed underwritten public offering of 6,500,000 shares of its common stock, consisting of 3,750,000 shares offered by Paragon and 2,750,000 shares offered by certain selling securityholders. Paragon and the selling securityholders also expect to grant the underwriters a 30-day option to purchase up to an additional 562,500 and 412,500 shares of common stock, respectively. The proposed offering is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed, or as to the actual size or terms of the offering.
By Paragon 28, Inc. · Via Business Wire · January 25, 2023

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or “P28”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced a range of its preliminary unaudited revenue for the fourth quarter and full-year ended December 31, 2022.
By Paragon 28, Inc. · Via Business Wire · January 10, 2023

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that Albert DaCosta, Chairman and CEO and Steve Deitsch, CFO will participate in a fireside chat at the 34th Annual Piper Sandler Healthcare Conference on Thursday, December 1, 2022 at 8:30 a.m. Eastern Time / 5:30 a.m. Pacific Time.
By Paragon 28, Inc. · Via Business Wire · November 16, 2022

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended September 30, 2022 and increased its 2022 net revenue guidance.
By Paragon 28, Inc. · Via Business Wire · November 10, 2022

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced it will report financial results for the third quarter 2022 after market close on Thursday, November 10, 2022. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time.
By Paragon 28, Inc. · Via Business Wire · October 27, 2022

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today that Albert DaCosta, Chairman and CEO, and Steve Deitsch, CFO, will be presenting at the Morgan Stanley 20th Annual Global Healthcare Conference. Mr. DaCosta and Mr. Deitsch will be hosting a fireside chat presentation on Tuesday, September 13th, 2022, at 3:30 p.m. Eastern Time / 12:30 p.m. Pacific Time.
By Paragon 28, Inc. · Via Business Wire · August 25, 2022

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced two changes to the composition of its Board of Directors.
By Paragon 28, Inc. · Via Business Wire · August 4, 2022

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended June 30, 2022 and increased its 2022 revenue guidance.
By Paragon 28, Inc. · Via Business Wire · August 3, 2022

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the second quarter 2022 after market close on Wednesday, August 3, 2022. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time.
By Paragon 28, Inc. · Via Business Wire · July 18, 2022

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today that Albert DaCosta, Chairman and CEO and Steve Deitsch, CFO will be presenting at The Canaccord Genuity 42nd Annual Growth Conference in Boston. The Canaccord Genuity fireside chat will be webcast live on August 11, 2022, at 1:00pm Eastern Time.
By Paragon 28, Inc. · Via Business Wire · July 18, 2022

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced its new nationwide mobile training lab tour, for convenient, on-site surgeon training and education.
By Paragon 28, Inc. · Via Business Wire · July 13, 2022
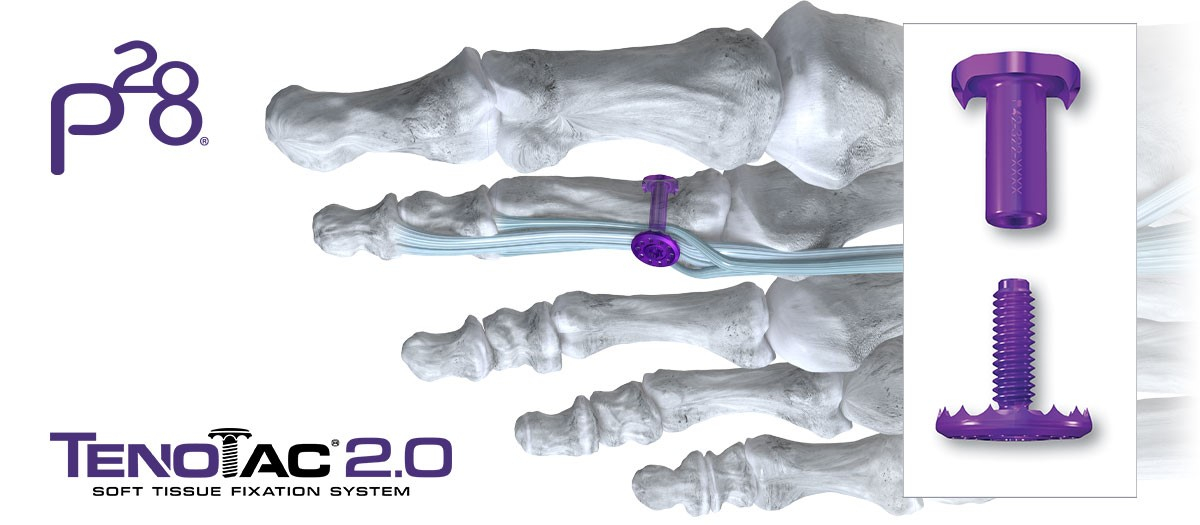
Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced further expansion of its hammertoe and soft-tissue portfolio with the launch of its TenoTac™ 2.0 Soft Tissue Fixation System. The TenoTac™ 2.0 Soft Tissue Fixation System uses a titanium threaded implant and was designed to allow for greater capture of soft tissue, streamlined tensioning, and improved fit to the bony surface.
By Paragon 28, Inc. · Via Business Wire · May 19, 2022

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today an expansion of its soft-tissue portfolio with the launch of its Grappler™ Suture Anchor System. The Grappler™ Suture Anchor System provides surgeons an alternative fixation option designed to limit implant migration and loss of tension for intraoperative tissue reattachment and fixation in the foot and ankle.
By Paragon 28, Inc. · Via Business Wire · May 17, 2022

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today reported financial results for the quarter ended March 31, 2022 and updated its 2022 revenue guidance.
By Paragon 28, Inc. · Via Business Wire · May 9, 2022
Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today an expansion of its hammertoe and soft-tissue portfolio with the launch of its Paratrooper™ Plantar Plate Repair System. The Paratrooper™ Plantar Plate System’s low profile, all-suture implant provides surgeons a new and innovative approach for plantar plate repair and forefoot deformities.
By Paragon 28, Inc. · Via Business Wire · April 27, 2022

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today the launch of its R3ACT™ Stabilization System, designed to be a simple solution that allows for multi-stage soft tissue healing following an acute or chronic syndesmotic injury to the ankle.
By Paragon 28, Inc. · Via Business Wire · April 19, 2022

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the first quarter 2022 after market close on Monday, May 9, 2022. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time.
By Paragon 28, Inc. · Via Business Wire · April 18, 2022

Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today the appointment of Meghan Scanlon to its Board of Directors. Ms. Scanlon has joined the Board’s Compensation and Quality, Technology and Regulatory Committees. Ms. Scanlon has more than 20 years of senior leadership experience with global medical device companies. She currently serves as senior vice president and president, Urology and Pelvic Health, for Boston Scientific Corporation and is a member of the Boston Scientific Executive Committee. Ms. Scanlon also is a member of the Boston Scientific Global Council for Inclusion, serving as the executive sponsor for the PRIDE employee resource group.
By Paragon 28, Inc. · Via Business Wire · March 28, 2022

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced it will report financial results for the fourth quarter and full year 2021 after market close on Tuesday, March 8, 2022. The company’s management will webcast a corresponding conference call beginning at 4:30 p.m. Eastern Time.
By Paragon 28, Inc. · Via Business Wire · February 17, 2022

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or “P28”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced the acquisition of Disior Oy., a leading three-dimensional analytics pre-operative planning software company based in Helsinki, Finland, focused on the complex foot and ankle anatomy.
By Paragon 28, Inc. · Via Business Wire · January 12, 2022

Paragon 28, Inc. (NYSE: FNA) (“Paragon 28” or “P28”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced a range of its preliminary unaudited revenue for the fourth quarter and full-year ended December 31, 2021.
By Paragon 28, Inc. · Via Business Wire · January 10, 2022

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced that the U.S. Food and Drug Administration (FDA) has given 501(k) marketing clearance to its R3ACT™ Stabilization System. The R3ACT™ Stabilization System, expected to commercially launch in early 2022, will complement Paragon 28’s comprehensive ankle fracture and soft tissue portfolio and further expands Paragon 28’s product offering in the foot and ankle space.
By Paragon 28, Inc. · Via Business Wire · December 29, 2021

Paragon 28, Inc. (NYSE: FNA) (“PARAGON”), a leading medical device company exclusively focused on the foot and ankle orthopedic market, announced today that Albert DaCosta, Chairman and CEO, and Steve Deitsch, CFO, will virtually present at the 24th Annual Needham Growth Conference on Monday, January 10th.
By Paragon 28, Inc. · Via Business Wire · December 16, 2021

Paragon 28, Inc. (NYSE: FNA) announced today that it has received a supplemental approval order from the U.S. Food and Drug Administration ("FDA") for the Patient Specific Talus Spacer. The supplemental approval order allows the Patient Specific Talus Spacer to be additively manufactured in titanium alloy with a titanium nitride coating.
By Paragon 28, Inc. · Via Business Wire · December 13, 2021
