Articles from Mind Medicine (MindMed) Inc.

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that Rob Barrow, Chief Executive Officer, will present at the 44th Annual J.P. Morgan Healthcare Conference in San Francisco on Wednesday, January 14, 2026 at 2:15 p.m. PT.
By Mind Medicine (MindMed) Inc. · Via Business Wire · December 18, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the issuance of inducement grants to two newly hired non-executive employees consisting of options to purchase an aggregate of 31,500 common shares of the Company (the "Options") with an effective grant date of December 15, 2025. The Options have an exercise price equal to the closing price of MindMed’s common shares on the date of the grant, and will vest over a four-year period with 25% vesting on the first anniversary of the date of the grant and the remaining 75% vesting in substantially equal monthly increments over the three-year period thereafter, subject to each employee’s continued employment.
By Mind Medicine (MindMed) Inc. · Via Business Wire · December 15, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the issuance of inducement grants to four newly hired non-executive employees consisting of options to purchase an aggregate of 182,100 common shares of the Company (the “Options”) with effective grant dates of November 17, 2025, November 24, 2025 and December 1, 2025, depending on the applicable employee’s respective start date. The Options have an exercise price equal to the closing price of MindMed’s common shares on the date of the respective grant, and will vest over a four-year period with 25% vesting on the first anniversary of the date of the grant and the remaining 75% vesting in substantially equal monthly increments over the three-year period thereafter, subject to each employee’s continued employment.
By Mind Medicine (MindMed) Inc. · Via Business Wire · December 1, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (the "Company" or "MindMed"), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today reported financial results for the third quarter ended September 30, 2025 and provided business updates.
By Mind Medicine (MindMed) Inc. · Via Business Wire · November 6, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that members of the Company’s management team will participate in the following investor conferences:
By Mind Medicine (MindMed) Inc. · Via Business Wire · November 5, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the issuance of inducement grants to three newly hired non-executive employees consisting of options to purchase an aggregate of 90,750 common shares of the Company (the "Options") with an effective grant date of November 3, 2025. The Options have an exercise price equal to the closing price of MindMed’s common shares on the date of the grant, and will vest over a four-year period with 25% vesting on the first anniversary of the date of the grant and the remaining 75% vesting in substantially equal monthly increments over the three-year period thereafter, subject to each employee’s continued employment.
By Mind Medicine (MindMed) Inc. · Via Business Wire · November 3, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the closing of its previously announced underwritten public offering of 21,131,250 common shares, without par value, which includes the exercise in full by the underwriters of their option to purchase an additional 2,756,250 common shares, at a public offering price of $12.25 per common share. All of the shares were offered by MindMed. The gross proceeds from this offering were approximately $259 million, before deducting underwriting discounts and commissions and offering expenses payable by MindMed.
By Mind Medicine (MindMed) Inc. · Via Business Wire · November 3, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that it will host a live webcast at 4:30 p.m. EST on Thursday, November 6, 2025 to report financial results for the third quarter ended September 30, 2025, and discuss recent business updates.
By Mind Medicine (MindMed) Inc. · Via Business Wire · October 30, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the pricing of an underwritten public offering of 18,375,000 common shares, without par value, at a public offering price of $12.25 per common share. The gross proceeds to MindMed from the offering, before deducting underwriting discounts, commissions, and other offering-related expenses, are expected to be approximately $225 million. In addition, MindMed has granted the underwriters an option for a period of 30 days to purchase up to an additional 2,756,250 common shares at the public offering price, less underwriting discounts and commissions. All of the common shares are being offered by MindMed.
By Mind Medicine (MindMed) Inc. · Via Business Wire · October 29, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that it intends to offer and sell, subject to market conditions, common shares and, to certain investors, pre-funded warrants to purchase common shares in an underwritten public offering. In addition, MindMed intends to grant the underwriters an option for a period of 30 days to purchase additional common shares at the public offering price, less underwriting discounts and commissions. All of the common shares and pre-funded warrants are being offered by MindMed.
By Mind Medicine (MindMed) Inc. · Via Business Wire · October 29, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the issuance of inducement grants to one newly hired non-executive employee consisting of an option to purchase 28,000 common shares of the Company (the "Option") with an effective grant date of October 14, 2025. The Option has an exercise price equal to the closing price of MindMed’s common shares on the date of the grant, and will vest over a four-year period with 25% vesting on the first anniversary of the date of the grant and the remaining 75% vesting in substantially equal monthly increments over the three-year period thereafter, subject to the employee’s continued employment.
By Mind Medicine (MindMed) Inc. · Via Business Wire · October 14, 2025

Researchers from Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (the "Company" or "MindMed"), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today presented results from a cross-sectional retrospective study of more than 75,000 respondents from the 2022 National Health and Wellness Survey at the Psych Congress 2025.
By Mind Medicine (MindMed) Inc. · Via Business Wire · September 19, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that it will host a live webcast at 4:30 p.m. EDT on Thursday, July 31, 2025 to report financial results for the first quarter ended June 30, 2025, and discuss recent business updates.
By Mind Medicine (MindMed) Inc. · Via Business Wire · July 24, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the issuance of inducement grants to six newly hired non-executive employees consisting of options to purchase an aggregate of 96,400 common shares of the Company (the "Options"), with effective grant dates of July 7, 2025 and July 14, 2025, depending on the applicable employee’s respective start date. The Options have an exercise price equal to the closing price of MindMed’s common shares on the date of the respective grant, and will vest over a four-year period with 25% vesting on the first anniversary of the date of the grant and the remaining 75% vesting in substantially equal monthly increments over the three-year period thereafter, subject to each employee’s continued employment.
By Mind Medicine (MindMed) Inc. · Via Business Wire · July 14, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that members of the Company’s management team will present at the 2025 RBC Capital Markets Global Healthcare Conference:
By Mind Medicine (MindMed) Inc. · Via Business Wire · May 13, 2025

Mind Medicine (MindMed) Inc. (Nasdaq: MNMD), (the "Company" or "MindMed"), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that it will host a live webcast at 8:00 a.m. ET on Thursday, May 8, 2025 to report financial results for the first quarter ended March 31, 2025, and discuss recent business updates.
By Mind Medicine (MindMed) Inc. · Via Business Wire · April 24, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (the "Company" or "MindMed"), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that the first patient has been dosed in its Phase 3 Emerge study evaluating MM120 ODT, a proprietary, pharmaceutically optimized form of LSD for the treatment of MDD. Emerge will evaluate the efficacy and safety of MM120 ODT 100 µg versus placebo and is expected to enroll approximately 140 participants in the United States. Emerge is the third Phase 3 study of MM120 ODT, with the Voyage and Panorama studies in GAD already underway.
By Mind Medicine (MindMed) Inc. · Via Business Wire · April 15, 2025

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (the "Company" or "MindMed"), a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that the first patient has been dosed in Panorama, its second Phase 3 study evaluating MM120 ODT, a proprietary, pharmaceutically optimized form of LSD for the treatment generalized anxiety disorder (GAD). The Panorama study will evaluate the efficacy and safety of MM120 ODT versus placebo, will be conducted in the United States and Europe, and is expected to enroll approximately 250 participants.
By Mind Medicine (MindMed) Inc. · Via Business Wire · January 30, 2025

Mind Medicine (MindMed) Inc. (Nasdaq: MNMD), (the "Company" or "MindMed"), a clinical-stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that it will be added to the Nasdaq Biotechnology Index (NBI), effective at market open on Monday, December 23, 2024.
By Mind Medicine (MindMed) Inc. · Via Business Wire · December 19, 2024
Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (the "Company" or "MindMed"), a clinical-stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that the first patient has been dosed in its Phase 3 Voyage study of MM120 ODT, a pharmaceutically optimized form of lysergide D-tartrate (LSD) for the treatment of GAD. Voyage is the first of two Phase 3 studies in GAD evaluating the efficacy and safety of MM120 ODT versus placebo and is expected to enroll approximately 200 participants in the United States. The Panorama study, the second Phase 3 trial, will be conducted in the U.S. and Europe and is on track to initiate in the first half of 2025.
By Mind Medicine (MindMed) Inc. · Via Business Wire · December 16, 2024

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (the "Company" or "MindMed"), a clinical-stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the appointment of Gregg A. Pratt, Ph.D., as Chief Regulatory and Quality Assurance Officer. Dr. Pratt will serve as a member of the Executive Committee and oversee the Company’s regulatory and quality functions, as well as its product registration strategies.
By Mind Medicine (MindMed) Inc. · Via Business Wire · November 18, 2024

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the pricing of an underwritten public offering of 9,285,511 common shares, without par value, at a public offering price of $7.00 per common share, and, to certain investors, pre-funded warrants to purchase 1,428,775 common shares at a price of $6.999 per pre-funded warrant, which represents the per share public offering price for the common shares less the $0.001 per share exercise price for each such pre-funded warrant. The gross proceeds to MindMed from the offering, before deducting underwriting discounts, commissions, and other offering-related expenses, are expected to be approximately $75 million.
By Mind Medicine (MindMed) Inc. · Via Business Wire · August 9, 2024

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that it intends to offer and sell, subject to market conditions, common shares and, to certain investors, pre-funded warrants to purchase common shares in an underwritten public offering. All of the common shares and pre-funded warrants are being offered by MindMed.
By Mind Medicine (MindMed) Inc. · Via Business Wire · August 9, 2024
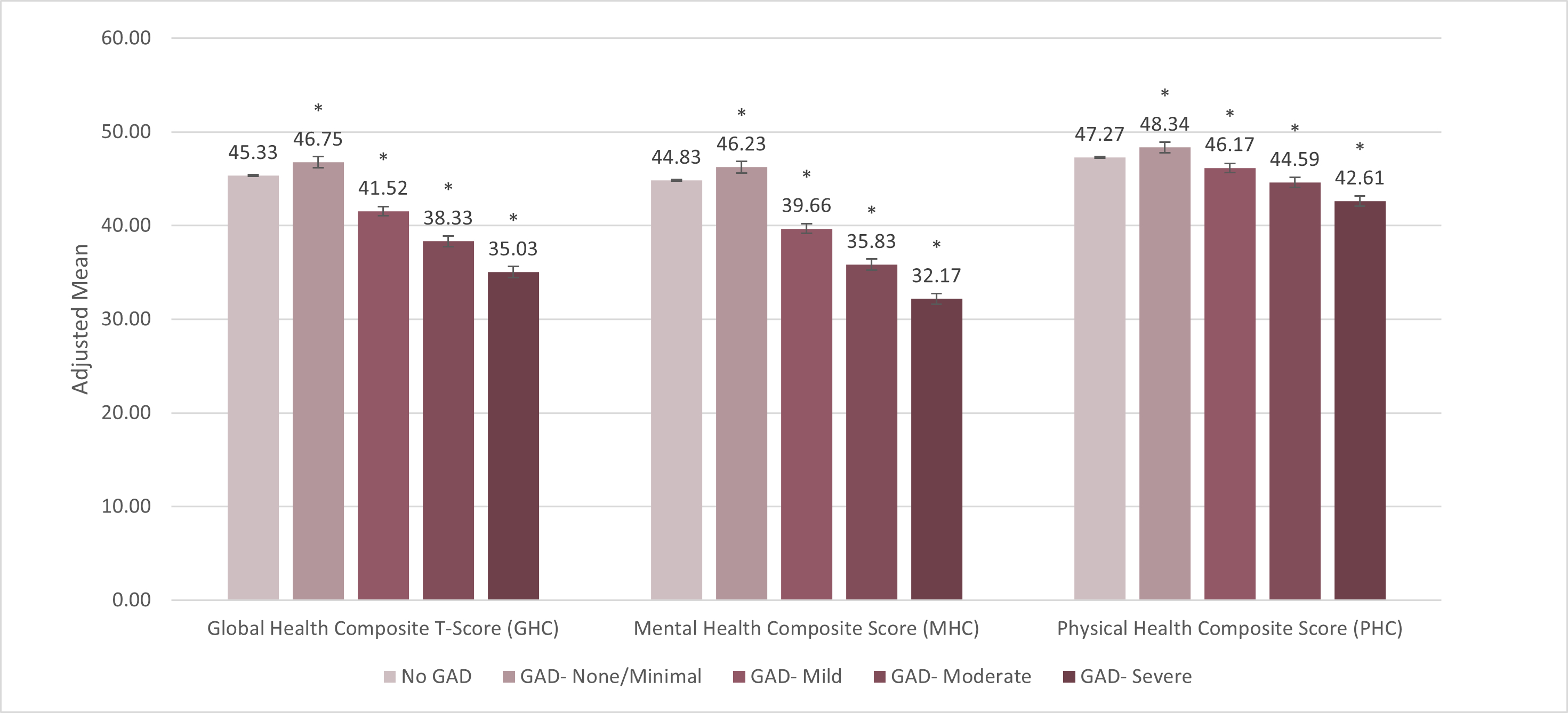
Researchers from Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today presented new studies highlighting the significant impact of GAD in the US at ISPOR 2024, the leading global conference for health economics and outcomes research (HEOR) being held this week in Atlanta.
By Mind Medicine (MindMed) Inc. · Via Business Wire · May 9, 2024

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (Cboe Canada: MMED) (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced the pricing of an underwritten offering of 16,666,667 common shares, no par value per share, at an offering price of $6.00 per common share. In addition, the Company has entered into share purchase agreements for a concurrent private placement of 12,500,000 common shares at a price of $6.00 per common share. All of the common shares are being sold by MindMed. Gross proceeds to MindMed from the underwritten offering and concurrent private placement, before deducting underwriting commissions, placement agent fees and other offering-related expenses, are expected to be approximately $175 million.
By Mind Medicine (MindMed) Inc. · Via Business Wire · March 7, 2024

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (Cboe Canada MMED), (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that FDA has granted breakthrough designation to its MM120 (lysergide d-tartrate) program for the treatment of generalized anxiety disorder (GAD). The Company also announced that its Phase 2b study of MM120 in GAD met its key secondary endpoint, and 12-week topline data demonstrated clinically and statistically significant durability of activity observed through Week 12.
By Mind Medicine (MindMed) Inc. · Via Business Wire · March 7, 2024

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the "Company" or "MindMed"), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today provided a corporate update and outlook for 2024.
By Mind Medicine (MindMed) Inc. · Via Business Wire · January 8, 2024

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, announced today that it plans to host a conference call on Thursday, November 2, 2023, at 4:30 p.m. ET to provide a corporate update and review the Company’s results for the quarter ended September 30, 2023.
By Mind Medicine (MindMed) Inc. · Via Business Wire · October 26, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (NEO: MMED) (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, announced today that it has completed enrollment and dosing in Study MMED008, the Company’s Phase 2b study evaluating MM-120 (lysergide D-tartrate) for the treatment of GAD.
By Mind Medicine (MindMed) Inc. · Via Business Wire · September 12, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (NEO: MMED) (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, announced today that members of the Company’s management team will participate in the following investor conferences:
By Mind Medicine (MindMed) Inc. · Via Business Wire · August 28, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that it has entered into a senior secured credit facility with K2 HealthVentures, a healthcare-focused specialty finance company.
By Mind Medicine (MindMed) Inc. · Via Business Wire · August 14, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the “Company” or "MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, announced today that it plans to host a conference call on Thursday, August 3, 2023, at 4:30 p.m. ET to provide a corporate update and review the Company’s results for the quarter ended June 30, 2023.
By Mind Medicine (MindMed) Inc. · Via Business Wire · July 31, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, announced today that members of the Company’s management team will participate in the H.C. Wainwright 4th Annual Neuropsychiatry Virtual Conference:
By Mind Medicine (MindMed) Inc. · Via Business Wire · June 22, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the “Company” or “MindMed”) today announced that leading proxy advisory firm Glass, Lewis & Co. (“Glass Lewis”) recommends shareholders vote for the election of ALL of MindMed’s six highly qualified director nominees to the Board of Directors (the “Board”) at the upcoming Annual Meeting of Shareholders (“Annual Meeting”), scheduled for June 15, 2023. Previously, leading proxy advisory firm Institutional Shareholder Services Inc. (“ISS”) also recommended in favor of all six of the Company’s director nominees.
By Mind Medicine (MindMed) Inc. · Via Business Wire · June 8, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the “Company” or “MindMed”) today announced that it has released an investor presentation in connection with its 2023 Annual Meeting of Shareholders. The presentation details how under the guidance of MindMed’s world-class Board of Directors the Company is executing against a well-defined plan to create value – with two Phase 2 clinical readouts for lead product candidate MM-120 (LSD D-tartrate) expected in late 2023. MindMed urges all shareholders to vote "FOR" the Company's six highly qualified director nominees using the WHITE universal proxy card at the upcoming Annual Meeting, scheduled for June 15, 2023.
By Mind Medicine (MindMed) Inc. · Via Business Wire · May 25, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED) (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that the Company has published a report by Greenleaf Health, Inc. (“Greenleaf”) setting forth an independent expert regulatory assessment of MindMed’s MM-120 (lysergide D-tartrate) development strategy. The analysis – led by the former Director and Deputy Director of the Office of New Drugs at the U.S. Food and Drug Administration (“FDA”) – focuses on MindMed’s clinical and regulatory development strategy for MM-120 and its ongoing Phase 2b trial in patients with generalized anxiety disorder (“GAD”). The findings support MindMed’s view that this trial is essential to the development of MM-120 in GAD and answers critical questions to inform a responsible development program. To read the full report, please visit: protectmindmed.com
By Mind Medicine (MindMed) Inc. · Via Business Wire · May 25, 2023

Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the “Company” or "MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, announced today that it plans to host a conference call on Thursday, May 4, 2023, at 4:30 p.m. ET to provide a corporate update and review the Company’s results for the quarter ended March 31, 2023.
By Mind Medicine (MindMed) Inc. · Via Business Wire · May 1, 2023
