Articles from Legend Biotech

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global leader in cell therapy, announced today results for the first time from the Phase 2 CARTITUDE-2 Cohort D study in multiple myeloma patients. Results showed patients with less than a complete response (CR) after front-line autologous stem cell transplant (ASCT) experienced deep and durable responses following a single infusion of CARVYKTI® (ciltacabtagene autoleucel; cilta-cel) with or without lenalidomide maintenance.1 These data were presented as an oral presentation at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting (Abstract #7505) and will also be shared as an encore oral presentation at the 2024 European Hematology Association (EHA) Congress (Abstract #S205).1 CARVYKTI® is the first and only B-cell maturation antigen (BCMA)-targeted therapy approved for the treatment of patients with relapsed/refractory multiple myeloma as early as after first relapse.
By Legend Biotech · Via Business Wire · June 3, 2024

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced it will participate in the upcoming investor conferences:
By Legend Biotech · Via Business Wire · June 2, 2023

Legend Biotech Corporation (“Legend Biotech” or the “Company”) (NASDAQ: LEGN), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced the appointment of Mythili Koneru, M.D., Ph.D. as the Company’s Chief Medical Officer. In this role, Dr. Koneru will be responsible for overseeing the Company’s clinical development and medical affairs programs.
By Legend Biotech · Via Business Wire · April 11, 2023

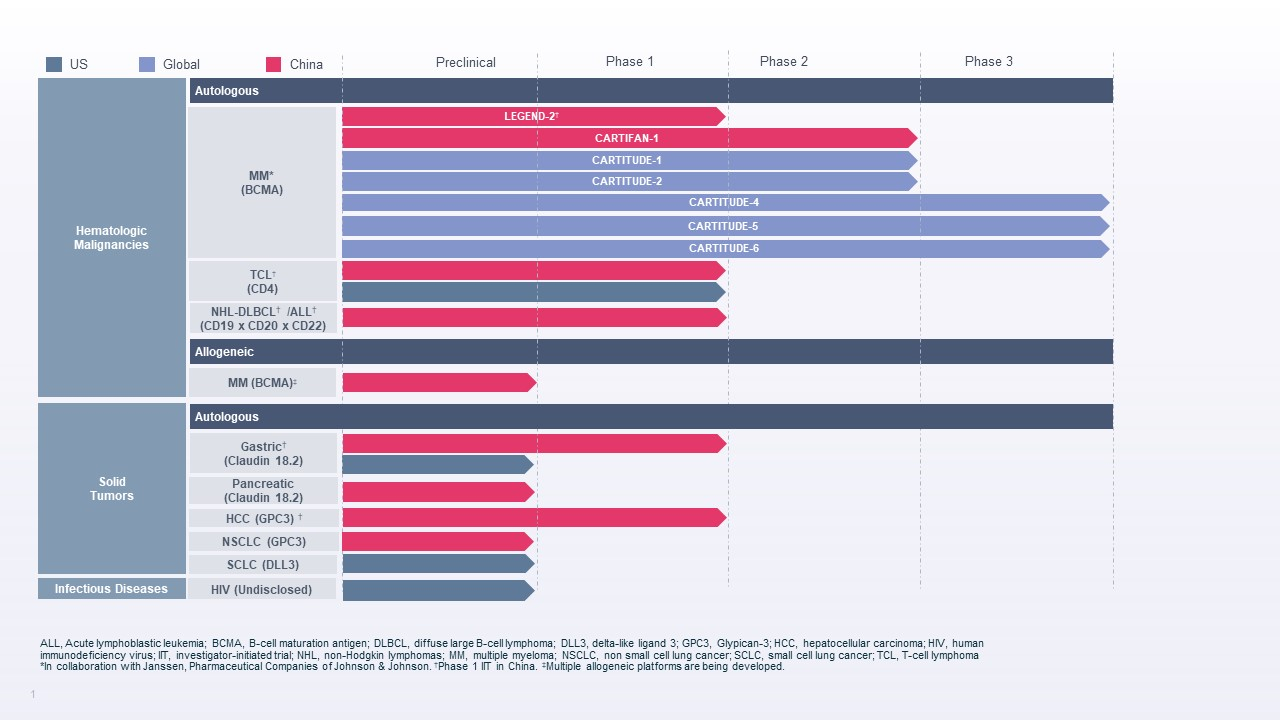
Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, announced today that results from the Phase 3 CARTITUDE-4 study showed that, at a median follow up of 16 months, cilta-cel reduced the risk of disease progression or death by 74 percent compared to standard of care regimens in adult patients with multiple myeloma who have received one to three prior lines of therapy and are refractory to lenalidomide (Hazard ratio [HR]=0.26 (95% CI, 0.18–0.38); P-value [P] <0.0001).1 The study data were featured in a press briefing and presented in an oral session at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting (Abstract #LBA106) and were presented in the New England Journal of Medicine. On Saturday, June 10, 2023, results will also be presented in a plenary session at the 2023 European Hematology Association (EHA) Hybrid Congress (Abstract #S100).
By Legend Biotech · Via Business Wire · June 5, 2023

Legend Biotech Corporation (“Legend Biotech” or the “Company”) (NASDAQ: LEGN), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced the formation of a strategic advisory board and the appointments of Michel Vounatsos, former CEO of Biogen Inc., and John Maraganore, PhD, former CEO of Alnylam Pharmaceuticals, as advisors. Mr. Vounatsos and Dr. Maraganore will work closely with Legend Biotech’s leadership team and Board of Directors to advise on strategic initiatives to advance the Company’s cell therapy platforms.
By Legend Biotech · Via Business Wire · April 3, 2023

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today reported its full year 2022 audited financial results.
By Legend Biotech · Via Business Wire · March 30, 2023

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced its participation in the upcoming investor conferences:
By Legend Biotech · Via Business Wire · February 24, 2023

Legend Biotech Corporation (“Legend Biotech” or the “Company”) (NASDAQ: LEGN), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, announced that it received notice from the Listing Qualifications Department of the Nasdaq Stock Market (“Nasdaq”) on February 17, 2023 indicating that the Company has regained compliance with Nasdaq Listing Rules 5250(c)(2).
By Legend Biotech · Via Business Wire · February 21, 2023

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, announced today that CARTITUDE-4, the Phase 3 study evaluating CARVYKTI® (ciltacabtagene autoleucel; cilta-cel) for the treatment of adult patients with relapsed and lenalidomide-refractory multiple myeloma, met its primary endpoint of showing a statistically significant improvement in progression-free survival (PFS) compared to standard therapy at the study’s first pre-specified interim analysis. The study has been unblinded following the recommendation of an independent data monitoring committee.
By Legend Biotech · Via Business Wire · January 27, 2023

Legend Biotech Corporation (“Legend Biotech” or the “Company”) (NASDAQ: LEGN), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, announced today that it received a notice (the “Notice”) from the Listing Qualifications Department of The Nasdaq Stock Market LLC (“Nasdaq”) on January 6, 2023 indicating that the Company is not currently in compliance with Nasdaq’s Listing Rules (the “Listing Rules”) due to the Company’s failure to file an interim balance sheet and income statement as of and for the quarter ended June 30, 2022 on Form 6-K with the Securities and Exchange Commission (“SEC”) . Pursuant to Listing Rule 5250(c)(2), the Company was required to file such Form 6-K no later than six months following the end of the quarter ended June 30, 2022, or December 31, 2022 (the “Due Date”). As of the date of this press release, the Company has not yet filed the required Form 6-K. This delay in filing such Form 6-K has resulted from the Company’s planned restatement of its audited financial statements as of and for the years ended December 31, 2021, December 31, 2020 and December 31, 2019 and unaudited interim financial information for the three months ended March 31, 2022, as previously reported by the Company on its Form 6-K dated October 20, 2022.
By Legend Biotech · Via Business Wire · January 9, 2023

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases today announced its participation in the 41st annual JP Morgan Healthcare Conference in San Francisco, CA.
By Legend Biotech · Via Business Wire · January 3, 2023

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, announced today that China’s National Medical Products Administration (NMPA) has formally accepted its New Drug Application (NDA) for ciltacabtagene autoleucel (cilta-cel).
By Legend Biotech · Via Business Wire · January 2, 2023

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, announced today that the U.S. Food and Drug Administration (FDA) has cleared Legend Biotech’s Investigational New Drug (IND) application to proceed with the clinical development of LB2102, an investigational, autologous chimeric antigen receptor T-cell (CAR-T) therapy for the treatment of adult patients with extensive stage small cell lung cancer (SCLC).
By Legend Biotech · Via Business Wire · November 21, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced its participation in the upcoming investor conferences:
By Legend Biotech · Via Business Wire · November 4, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced its participation in the upcoming investor conferences:
By Legend Biotech · Via Business Wire · August 19, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced the appointment of three new directors to its Board: Mr. Tomas Heyman as Class I director of Legend Biotech, Dr. Fangliang “Frank” Zhang as Class II director of Legend Biotech, and Dr. Li Mao as Class III director of Legend Biotech. Mr. Heyman and Dr. Mao were identified by the Board as meeting the independence requirements of the Listing Rules of The Nasdaq Stock Market LLC. Ms. Ye “Sally” Wang also resigned as Chair, effective August 2, 2022, but will remain on the Board as a director and in her committee assignments. Dr. Frank Zhang, one of the newly appointed directors, was elected Chairman of the Board of Directors.
By Legend Biotech · Via Business Wire · August 4, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, presented today new and updated results from the CARTITUDE clinical development program studying ciltacabtagene autoleucel (cilta-cel) in the treatment of multiple myeloma at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting. Earlier data from the CARTITUDE-1 study supported recent regulatory approvals for CARVYKTI™ by the U.S. Food and Drug Administration and the European Commission, and ongoing results from the multi-cohort CARTITUDE-2 study are being used to inform future trials of CARVYKTI™ treatment in multiple patient populations and treatment settings.
By Legend Biotech · Via Business Wire · June 4, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced that the U.S. Food and Drug Administration (FDA) has cleared its investigational new drug (IND) application to evaluate LB1908 in a Phase 1 clinical trial in the United States. LB1908 is an investigational, autologous chimeric antigen receptor T-cell (CAR-T) therapy selectively targeting Claudin 18.2 through a high-affinity VHH antibody for the treatment of adults with relapsed or refractory gastric, esophageal (including gastro-esophageal junction) or pancreatic cancers. Claudin18.2 is a tight junction protein commonly expressed in patients with these cancer subtypes.1
By Legend Biotech · Via Business Wire · June 3, 2022
Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today reported its first quarter 2022 unaudited financial results.
By Legend Biotech · Via Business Wire · June 1, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced that it will host an investor event during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting. Taking place on Sunday, June 5 at 6pm Central Time, the meeting will feature Dr. Sundar Jagannath, Professor of Medicine, Hematology and Medical Oncology at Mount Sinai School of Medicine and Director of the Multiple Myeloma Program at Mount Sinai Hospital.
By Legend Biotech · Via Business Wire · May 31, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced that the European Commission (EC) has granted conditional marketing authorization of CARVYKTI® (ciltacabtagene autoleucel; cilta-cel) for the treatment of adults with relapsed and refractory multiple myeloma (RRMM) who have received at least three prior therapies, including a proteasome inhibitor (PI), an immunomodulatory agent (IMiD) and an anti-CD38 antibody, and have demonstrated disease progression on the last therapy. Legend Biotech entered into an exclusive worldwide license and collaboration agreement with Janssen Biotech, Inc. (Janssen) to develop and commercialize cilta-cel in December 2017.
By Legend Biotech · Via Business Wire · May 26, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced that eight company-sponsored abstracts were accepted at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting and the European Hematology Association’s (EHA) 2022 Hybrid Congress.
By Legend Biotech · Via Business Wire · May 18, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced the promotion of Lori Macomber, CPA, to Chief Financial Officer (CFO), effective immediately. Ms. Macomber assumes the role from Dr. Ying Huang, who has been both CFO and Chief Executive Officer (CEO) since September 2020. Dr. Huang will continue to serve as CEO of Legend Biotech.
By Legend Biotech · Via Business Wire · May 9, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced its participation in the upcoming investor conferences:
By Legend Biotech · Via Business Wire · May 6, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced the achievement of a $50 million milestone under its collaboration agreement with Janssen Biotech, Inc. (Janssen) for ciltacabtagene autoleucel (cilta-cel), now marketed in the United States under the brand name CARVYKTI™. Cilta-cel is a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell (CAR-T) therapy.
By Legend Biotech · Via Business Wire · April 21, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended Janssen Pharmaceutica NV’s marketing authorization of CARVYKTI® (ciltacabtagene autoleucel; cilta-cel) for the treatment of adults with relapsed and refractory multiple myeloma who have received at least three prior therapies, including an immunomodulatory agent, a proteasome inhibitor and an anti-CD38 antibody and have demonstrated disease progression on the last therapy.
By Legend Biotech · Via Business Wire · March 25, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, was named Newcomer of the Year at the tenth annual Foreign Investment Trophy ceremony hosted by Flanders Investment & Trade (FIT).
By Legend Biotech · Via Business Wire · March 23, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies, today reported unaudited financial results for the fourth quarter of 2021.
By Legend Biotech · Via Business Wire · March 18, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global, clinical-stage biotechnology company developing and manufacturing novel therapies, today in conjunction with an announcement to be issued by Legend Biotech’s majority shareholder, GenScript Biotech Corporation, pursuant to the rules of The Stock Exchange of Hong Kong Limited, announced preliminary, unaudited financial results for the year ended December 31, 2021.
By Legend Biotech · Via Business Wire · February 18, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global, clinical-stage biotechnology company developing and manufacturing novel therapies, today announced that on Friday, February 11, 2022, the company was informed by the U.S Food and Drug Administration (FDA) via e-mail communication that its Phase 1 clinical trial for LB1901 has been placed on clinical hold. LB1901 is the company’s investigational autologous chimeric antigen receptor T-cell (CAR-T) therapy targeting malignant CD4+ T-cells for the treatment of adults with relapsed or refractory T-cell lymphoma (TCL). The FDA indicated they will provide an official clinical hold letter to Legend Biotech by March 11, 2022.
By Legend Biotech · Via Business Wire · February 15, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global, clinical-stage biotechnology company developing and manufacturing novel therapies, has achieved two milestones under its collaboration agreement with Janssen Biotech, Inc. for ciltacabtagene autoleucel (cilta-cel), resulting in aggregate payments to Legend Biotech of $50 million. Cilta-cel is a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell (CAR-T) therapy.
By Legend Biotech · Via Business Wire · February 11, 2022

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global, clinical-stage biotechnology company developing and manufacturing novel therapies, announced today new and updated results from the CARTITUDE clinical development program studying ciltacabtagene autoleucel (cilta-cel) in the treatment of multiple myeloma, which were presented at the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition. Cilta-cel is an investigational B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T cell (CAR-T) therapy being studied as a one-time treatment for multiple myeloma.
By Legend Biotech · Via Business Wire · December 13, 2021

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global, clinical-stage biotechnology company developing and manufacturing novel therapies, today announced that it will host a hybrid event featuring key opinion leaders (KOLs) in multiple myeloma on Monday, December 13 at 8pm ET.
By Legend Biotech · Via Business Wire · December 7, 2021

Legend Biotech Corporation (NASDAQ: LEGN), a global, clinical-stage biotechnology company developing and manufacturing novel therapies, announced today the submission of a New Drug Application (NDA) to the Japanese Ministry of Health, Labour and Welfare (MHLW) for ciltacabtagene autoleucel (cilta-cel) by its collaboration partner, Janssen Pharmaceutical K.K. (Janssen). Cilta-cel is an investigational B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR)-T cell therapy for the treatment of adults with relapsed or refractory multiple myeloma who have received at least three prior therapies, including a proteasome inhibitor (PI), an immunomodulatory agent (IMiD), and an anti-CD38 antibody.
By Legend Biotech · Via Business Wire · December 6, 2021

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global, clinical-stage biotechnology company developing and manufacturing novel therapies, today reported its 2021 third quarter unaudited financial results.
By Legend Biotech · Via Business Wire · November 16, 2021

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global, clinical-stage biotechnology company developing and manufacturing novel therapies, today announced that the U.S. Food and Drug Administration has extended the Prescription Drug User Fee Act (PDUFA) target date for ciltacabtagene autoleucel (cilta-cel) to February 28, 2022. Cilta-cel is a BCMA-directed chimeric antigen receptor T cell (CAR-T) therapy being investigated for the treatment of adults with relapsed and/or refractory multiple myeloma. The Biologics License Application (BLA) was submitted by Legend Biotech’s collaboration partner Janssen Biotech, Inc. (Janssen).
By Legend Biotech · Via Business Wire · November 1, 2021

Legend Biotech Corporation (NASDAQ: LEGN), a global clinical-stage biopharmaceutical company engaged in the discovery and development of novel cell therapies for oncology and other indications, will host its first Research & Development Day on Monday, October 18, commencing at 10:00 a.m. (Eastern Time), at Andaz 5th Avenue in New York.
By Legend Biotech · Via Business Wire · September 22, 2021

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global clinical-stage biopharmaceutical company engaged in the discovery and development of novel cell therapies for oncology and other indications, has announced the start of a Phase 1 clinical trial in the United States for LB1901, an investigational autologous CD4-targeted chimeric antigen receptor T-cell (CAR-T) therapy for the treatment of adults with relapsed or refractory peripheral T-cell lymphoma (PTCL) or cutaneous T-cell lymphoma (CTCL). LB1901 targets CD4, a surface membrane glycoprotein uniformly expressed in most TCL subtypes. The trial follows the U.S. Food and Drug Administration (FDA) clearance of the Investigational New Drug (IND) application submitted by Legend Biotech.
By Legend Biotech · Via Business Wire · September 13, 2021

Legend Biotech Corporation (NASDAQ: LEGN), a global clinical-stage biopharmaceutical company engaged in the discovery and development of novel cell therapies for oncology and other indications, will participate in the 19th Annual Morgan Stanley Virtual Global Healthcare Conference on Friday, September 10. Ying Huang, Chief Executive Officer and Chief Financial Officer, will represent the Company in a session scheduled at 8:45 a.m. (Eastern Time).
By Legend Biotech · Via Business Wire · August 30, 2021

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global clinical-stage biopharmaceutical company engaged in the discovery and development of novel cell therapies for oncology and other indications, today reported its 2021 second quarter unaudited financial results.
By Legend Biotech · Via Business Wire · August 23, 2021

Legend Biotech Corporation (Legend Biotech, NASDAQ: LEGN), a global clinical-stage biopharmaceutical company engaged in the discovery and development of novel cell therapies for oncology and other indications, announced the establishment of a state-of-the-art manufacturing facility in Belgium, as part of a joint investment with Janssen Pharmaceutica NV (Janssen), to expand global manufacturing capacity of innovative cellular therapies.
By Legend Biotech · Via Business Wire · June 22, 2021

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global clinical-stage biopharmaceutical company engaged in the discovery and development of novel cell therapies for oncology and other indications, announced today that new and updated results for ciltacabtagene autoleucel (cilta-cel), an investigational BCMA-directed CAR-T therapy for the treatment of relapsed or refractory multiple myeloma (RRMM), will be featured at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting and at the European Hematology Association (EHA) Virtual Congress.
By Legend Biotech · Via Business Wire · June 1, 2021

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global clinical-stage biopharmaceutical company engaged in the discovery and development of novel cell therapies for oncology and other indications, has announced that the U.S. Food and Drug Administration (FDA) has accepted for priority review the Biologics License Application (BLA) submitted by Janssen Biotech, Inc. (Janssen) for ciltacabtagene autoleucel (cilta-cel), an investigational B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T cell (CAR-T) therapy. The Prescription Drug User Fee Act (PDUFA) target action date has been set for November 29, 2021.
By Legend Biotech · Via Business Wire · May 26, 2021

Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global clinical-stage biopharmaceutical company engaged in the discovery and development of novel cell therapies for oncology and other indications, today announced that 15 abstracts have been accepted at the upcoming 2021 American Society of Clinical Oncology (ASCO) Annual Meeting and the European Hematology Association’s (EHA) 2021 Virtual Congress, including new and updated data from the CARTITUDE clinical development program being led by Janssen Research & Development, LLC (Janssen) for the investigational B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T cell (CAR-T) therapy, ciltacabtagene autoleucel (cilta-cel).
By Legend Biotech · Via Business Wire · May 12, 2021
