Articles from Lantern Pharma Inc.

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company leveraging artificial intelligence to accelerate oncology drug discovery and development, today announced that CEO and President Panna Sharma will present at the 7th Glioblastoma Drug Development Summit, February 17–19, 2026, in Boston, MA.
By Lantern Pharma Inc. · Via Business Wire · February 10, 2026

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using artificial intelligence to transform the cost, pace, and timeline of oncology drug discovery and development, today announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to LP-284 for the treatment of soft tissue sarcomas.
By Lantern Pharma Inc. · Via Business Wire · January 20, 2026

Lantern Pharma Inc. (NASDAQ: LTRN), a pioneer in AI-driven precision oncology and computational therapeutic development, today announced the establishment of an A.I. Center of Excellence and Advanced Agentic Labs in Bengaluru, India. This strategic initiative represents a critical inflection point in Lantern's evolution—transitioning from pioneering AI-enabled drug discovery in cancer to industrializing those capabilities at global scale for the broader drug development community.
By Lantern Pharma Inc. · Via Business Wire · January 12, 2026

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biotechnology company using artificial intelligence and genomics to transform oncology drug development, announced additional details and clinical insights from its completed Phase 1a dose-escalation study of LP-184 as well as highlights from its recent webinar. The clinical trial demonstrated encouraging durable disease control in 63 heavily pre-treated patients with advanced solid tumors, many of which had DNA damage repair (DDR) pathway deficiencies. The clinical trial met all primary endpoints for safety, tolerability, and established a clear recommended phase 2 dose (RP2D).
By Lantern Pharma Inc. · Via Business Wire · December 3, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company leveraging its proprietary RADR® artificial intelligence (AI) and machine learning (ML) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced operational highlights and financial results for the third quarter 2025 ended September 30, 2025, and provided an update on its portfolio of AI-driven drug candidates and AI platform, RADR®.
By Lantern Pharma Inc. · Via Business Wire · November 13, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its third quarter 2025 operating and financial results webcast on Thursday, November 13, 9:00 a.m. Eastern Time / 6:00 a.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · November 6, 2025

Lantern Pharma Inc. (NASDAQ: LTRN)— Lantern today announced it will present two commercially deployed AI research platforms at the inaugural AI for Biology and Medicine (AI4BM) symposium at the University of North Texas. The symposium, hosted by Dr. Serdar Bozdag and the newly established Center for Computational Life Sciences, brings together leading researchers advancing the intersection of artificial intelligence and biomedicine.
By Lantern Pharma Inc. · Via Business Wire · October 30, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company leveraging its proprietary RADR® artificial intelligence platform to transform oncology drug discovery and development, today announced the presentation of clinical data from its ongoing Phase 1 trial of LP-284 at the 25th Annual Lymphoma, Leukemia & Myeloma (LL&M) Congress, held October 14-17, 2025, in New York City. The presentation featured a confirmed complete metabolic response in a 41-year-old patient with aggressive Grade 3 non-germinal center B-cell diffuse large B-cell lymphoma (DLBCL) who experienced rapid disease progression following four prior treatment regimens, including CAR-T cell therapy and bispecific antibody therapy.
By Lantern Pharma Inc. · Via Business Wire · October 28, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company dedicated to developing cancer therapies and transforming the cost, pace, and timeline of oncology drug discovery and development, today announced that Lantern management will be presenting at the ThinkEquity Conference on Thursday, October 30, 2025, at 11:30 a.m. ET at the Mandarin Oriental in New York, NY.
By Lantern Pharma Inc. · Via Business Wire · October 24, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a leading artificial intelligence (AI)-driven oncology company leveraging its proprietary RADR® platform to accelerate targeted cancer therapies, today announced the successful completion of its Phase 1a clinical trial (NCT05933265) for LP-184. The trial met all primary endpoints, demonstrating a favorable safety and pharmacokinetic (PK) profile, and early signs of antitumor activity. Enrollment is complete, with several patients continuing treatment due to ongoing clinical benefit.
By Lantern Pharma Inc. · Via Business Wire · September 16, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an AI-driven clinical -stage oncology company developing targeted therapies for cancer that are being advanced using its proprietary computational biology and machine learning platform, today announced the successful completion of a Type C meeting with the U.S. Food and Drug Administration (FDA). The meeting provided critical guidance on the regulatory pathway and trial design for a planned pediatric trial focused on CNS cancers, including Atypical Teratoid Rhabdoid Tumor (ATRT).
By Lantern Pharma Inc. · Via Business Wire · September 3, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company leveraging its proprietary RADR® artificial intelligence (AI) and machine learning (ML) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced operational highlights and financial results for the second quarter 2025 ended June 30, 2025, and provided an update on its portfolio of AI-driven drug candidates and AI platform, RADR®.
By Lantern Pharma Inc. · Via Business Wire · August 13, 2025

Starlight Therapeutics, a wholly owned subsidiary of Lantern Pharma Inc. (NASDAQ: LTRN), today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for a Phase Ib/2a clinical trial to evaluate STAR-001 (LP-184) in combination with spironolactone for patients with glioblastoma multiforme (GBM) at first progression.
By Lantern Pharma Inc. · Via Business Wire · August 6, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a pioneering artificial intelligence company transforming oncology drug discovery and development, today announced the public release of its AI module for predicting blood-brain barrier (BBB) permeability of small molecules with unprecedented accuracy and scalability – predictBBB.ai™. This critical advancement addresses one of pharmaceutical development's most persistent challenges—that only 2-6% of small-molecule drugs can successfully cross the blood-brain barrier—while establishing new industry benchmarks for computational drug discovery platforms.
By Lantern Pharma Inc. · Via Business Wire · August 4, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted cancer therapies using its proprietary RADR® AI platform, today announced the successful completion of targeted enrollment for its Phase 2 HARMONIC™ clinical trial in Japan. The company enrolled 10 patients ahead of schedule across five clinical sites in Japan, including the National Cancer Center Japan.
By Lantern Pharma Inc. · Via Business Wire · July 31, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using artificial intelligence, machine learning and genomic data to transform the cost, pace and timeline of oncology drug discovery and development, today announced the appointment of Lee T. Schalop, MD, to its Board of Directors.
By Lantern Pharma Inc. · Via Business Wire · July 28, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage oncology company leveraging its proprietary RADR® artificial intelligence (AI) platform to systematically transform drug discovery paradigms, today announces that a heavily pretreated patient with aggressive Grade 3 non-germinal center B-cell diffuse large B-cell lymphoma (DLBCL) achieved a complete metabolic response in the ongoing Phase 1 clinical trial of LP-284. This represents the first complete response observed with LP-284 and displays profound clinical activity in one of the most therapeutically challenging hematologic cancers.
By Lantern Pharma Inc. · Via Business Wire · July 23, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage oncology company leveraging its proprietary RADR® artificial intelligence (AI) platform to accelerate drug discovery, today announced that the European Patent Office (EPO) has issued a notice of allowance for a composition of matter patent covering its drug candidate LP-284. This patent, expected to be granted in the coming months with exclusivity through early 2039, strengthens Lantern's global intellectual property (IP) portfolio and supports the development and commercialization path for LP-284, a novel therapy in development for relapsed or refractory non-Hodgkin's lymphoma (NHL), including mantle cell lymphoma (MCL) and high-grade B-cell lymphomas (HGBL).
By Lantern Pharma Inc. · Via Business Wire · July 21, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a pioneering artificial intelligence (AI) company transforming oncology drug discovery and development, today announced the launch of an innovative AI-powered module within its proprietary RADR® platform, designed to predict the activity and efficacy of combination regimens involving DNA-damaging agents (DDAs) and DNA damage response inhibitors (DDRis) in clinical cancer treatment. With the global market for combination cancer therapies projected to exceed $50 billion by 2030, growing at a CAGR of 8.5%, this module represents a significant advancement in precision oncology, enabling faster, more cost-effective development of tailored therapeutic regimens. Leveraging this AI-driven framework, Lantern Pharma has successfully architected and achieved FDA clearance for a Phase 1B/2 clinical trial in triple-negative breast cancer (TNBC), focusing on a novel DDA-DDRi combination regimen with promising preclinical efficacy.
By Lantern Pharma Inc. · Via Business Wire · July 15, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted cancer therapies using its proprietary RADR® AI platform, today announces remarkable clinical observations for a patient in Lantern’s Phase 2 HARMONIC™ clinical trial. A 70-year-old never-smoker with advanced non-small cell lung cancer (NSCLC) has achieved a durable complete response in their target cancer lesions following treatment with LP-300 in combination with standard-of-care chemotherapy. Importantly, the patient continues to show sustained survival benefits after nearly two years.
By Lantern Pharma Inc. · Via Business Wire · June 16, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company leveraging advanced AI and machine learning to transform the cost, pace, and timeline of oncology drug development, today announced promising preclinical data for LP-184 in atypical teratoid rhabdoid tumors (ATRT), a rare and aggressive pediatric brain cancer. The results were presented by Dr. Eric Raabe of Johns Hopkins University School of Medicine at the Society for Neuro-Oncology's 8th Biennial Pediatric Neuro-Oncology Conference held May 15-17, 2025, in San Diego, California.
By Lantern Pharma Inc. · Via Business Wire · May 29, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company leveraging its proprietary RADR® artificial intelligence (AI) and machine learning (ML) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced operational highlights and financial results for the first quarter 2025 ended March 31, 2025, and provided an update on its portfolio of AI-driven drug candidates, the RADR® platform for precision oncology drug development enhancements, and other operational progress.
By Lantern Pharma Inc. · Via Business Wire · May 15, 2025

Lantern Pharma Inc. (Nasdaq: LTRN), an artificial intelligence company developing targeted and transformative cancer therapies using its proprietary AI platform, RADR®, today announced that the U.S. Food and Drug Administration (FDA) has cleared the amendment to its Investigational New Drug (IND) application to initiate a Phase 1b/2 clinical trial of LP-184 in a genomically defined patient population of non-small cell lung cancer (NSCLC) where there is a need to improve patient outcomes.
By Lantern Pharma Inc. · Via Business Wire · May 12, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its first quarter 2025 operating and financial results webcast on Thursday, May 15, 9:00 a.m. Eastern Time / 6:00 a.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · May 8, 2025

Lantern Pharma Inc. (Nasdaq: LTRN), an artificial intelligence company developing targeted and transformative cancer therapies using its proprietary AI platform, RADR®, today announced that it has received clearance of its Investigational New Drug Application (IND) from the U.S. Food and Drug Administration (FDA) for a Phase 1b/2 clinical trial for LP-184 in Triple Negative Breast Cancer. This achievement builds on the previous regulatory momentum including Orphan Drug Designation in 2023 and Fast Track Designation in 20241.
By Lantern Pharma Inc. · Via Business Wire · May 5, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company leveraging its proprietary RADR® artificial intelligence (AI) and machine learning (ML) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced financial results for the fourth quarter and full year ended December 31, 2024, and provided an update on its portfolio of AI-driven drug candidates, the RADR® platform for precision oncology drug development enhancements, and other operational progress.
By Lantern Pharma Inc. · Via Business Wire · March 27, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its fourth quarter and fiscal year 2024 operating and financial results webcast on Thursday, March 27, 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · March 20, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company dedicated to developing cancer therapies and transforming the cost, pace, and timeline of oncology drug discovery and development, today announced the publication of its PCT patent application (PCT/US2024/019851) covering a novel machine learning solution for predicting blood-brain barrier (BBB) permeability. The application received a favorable PCT search report indicating no significant prior art, substantially strengthening its path to approval.
By Lantern Pharma Inc. · Via Business Wire · February 19, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company dedicated to developing cancer therapies and transforming the cost, pace, and timeline of oncology drug discovery and development, today announced advancements in the application of its RADR® AI platform to accelerate and optimize the development of antibody-drug conjugates (ADCs). The global ADC market is projected to reach $30.4 billion by 2028, growing at a CAGR of 41.7%, with several recently approved ADCs achieving blockbuster status with annual sales exceeding $1 billion. Major biotech and pharmaceutical companies have recently completed ADC-focused acquisitions valued at over $10 billion, highlighting the sector's growing strategic importance. Lantern Pharma is actively advancing multiple ADC candidates through preclinical development, including a promising collaboration with the prestigious MAGICBULLET::Reloaded Initiative at the University of Bielefeld in Germany.
By Lantern Pharma Inc. · Via Business Wire · January 27, 2025

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted cancer therapies using its proprietary RADR® AI platform, today announced that the first patient has been enrolled and dosed in Taiwan for its Phase 2 HARMONIC™ clinical trial evaluating LP-300 in never-smoker patients with non-small cell lung cancer (NSCLC) who have progressed after receiving treatment with tyrosine kinase inhibitors (TKIs).
By Lantern Pharma Inc. · Via Business Wire · December 9, 2024
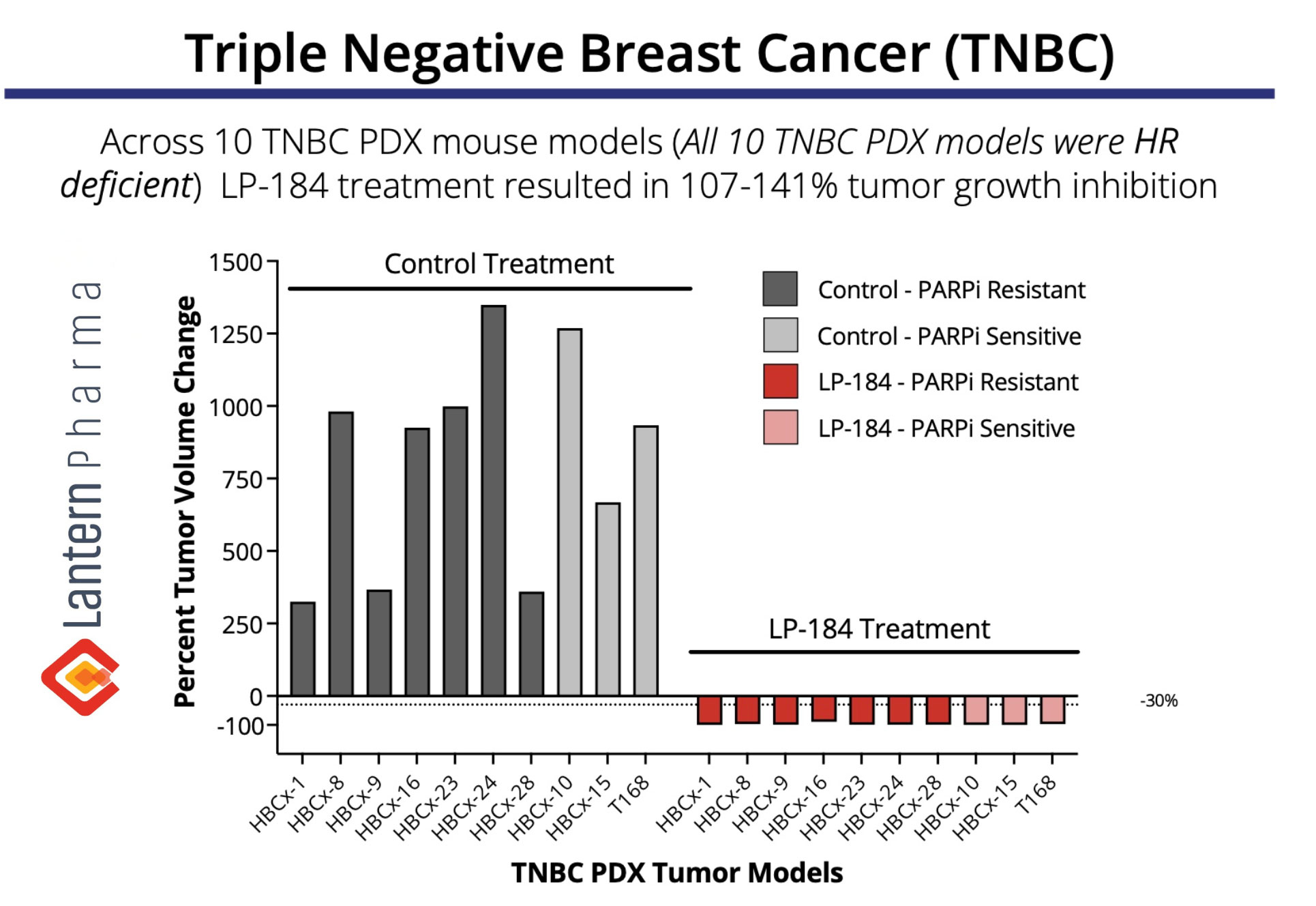
Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company dedicated to developing cancer therapies and transforming the cost, pace, and timeline of oncology drug discovery and development, today announced that the FDA has granted Fast Track Designation for investigational drug candidate, LP-184, for treatment of Triple Negative Breast Cancer (TNBC). This marks the second Fast Track Designation received for LP-184 in 2024, following its designation for Glioblastoma in October.
By Lantern Pharma Inc. · Via Business Wire · December 3, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence company transforming oncology drug development, and its wholly-owned subsidiary Starlight Therapeutics, focused exclusively on CNS and brain cancers, today announced the presentation of new preclinical data and Phase 1b trial design for LP-184 (to be developed as STAR-001 for CNS indications) in glioblastoma at the Society for Neuro-Oncology (SNO) 2024 Annual Meeting in Houston, Texas.
By Lantern Pharma Inc. · Via Business Wire · November 26, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted cancer therapies using its proprietary RADR® AI platform, today announced that the first patient has been dosed – as part of the expansion cohort – in Japan for its Phase 2 HARMONIC™ clinical trial evaluating LP-300 in never-smoker patients with non-small cell lung cancer (NSCLC) who have progressed after receiving treatment with tyrosine kinase inhibitors (TKIs).
By Lantern Pharma Inc. · Via Business Wire · November 19, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, today announced operational highlights and financial results for the third quarter 2024, ending September 30, 2024.
By Lantern Pharma Inc. · Via Business Wire · November 7, 2024

Starlight Therapeutics, a wholly-owned subsidiary of Lantern Pharmaceuticals (NASDAQ: LTRN), formed to develop transformative CNS cancer treatments with AI-powered innovation, announced today the formation of a Scientific Advisory Board (“SAB”) to support the development of lead compound STAR-001. The inaugural SAB will work closely with Starlight’s management team to provide strategic guidance and critical expert insights into the development of STAR-001 in CNS and brain tumors for both children and adults.
By Lantern Pharma Inc. · Via Business Wire · November 5, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its third quarter 2024 operating and financial results webcast on Thursday, November 7, 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · October 31, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company dedicated to developing cancer therapies and transforming the cost, pace, and timeline of oncology drug discovery and development, today announced that Lantern management will be presenting at the ThinkEquity Conference on Wednesday, October 30, 2024, at 10:30 a.m. ET at the Mandarin Oriental in New York, NY.
By Lantern Pharma Inc. · Via Business Wire · October 23, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company dedicated to developing cancer therapies and transforming the cost, pace, and timeline of oncology drug discovery and development, today announced that they will be participating in and hosting two webinars that are open to the public during October.
By Lantern Pharma Inc. · Via Business Wire · October 21, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company dedicated to developing cancer therapies and transforming the cost, pace, and timeline of oncology drug discovery and development, today announced that the FDA has granted Fast Track Designation for investigational drug candidate, LP-184, for treatment of Glioblastoma. LP-184 is currently in a Phase 1A clinical trial designed to evaluate the safety and tolerability of the synthetically lethal investigational drug candidate in a broad range of solid tumors, including Glioblastoma (GBM). LP-184 was optimized and advanced in part with Lantern’s AI platform, RADR®, to aid in the validation of mechanisms that could be exploited in the clinical setting to eradicate challenging cancers, and uncover insights in targeted patient populations. RADR® is Lantern’s AI platform for cancer therapy discovery, development and rescue with over 100 billion data points and aiding in the development of both Lantern’s portfolio and development initiatives with Lantern’s collaborators.
By Lantern Pharma Inc. · Via Business Wire · October 15, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, announced today that the company has been granted three rare pediatric disease designations (RPDD) by the FDA. Lantern was granted these rare pediatric disease designations in: malignant rhabdoid tumors (MRT), rhabdomyosarcoma (RMS), and hepatoblastoma.
By Lantern Pharma Inc. · Via Business Wire · September 23, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, today announced operational highlights and financial results for the second quarter 2024, ending June 30, 2024.
By Lantern Pharma Inc. · Via Business Wire · August 8, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its second quarter 2024 operating and financial results webcast on Thursday, August 8, 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · August 1, 2024

Lantern Pharma Inc., (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, today announced that the Japan Patent Office (JPO) has issued a Certificate of Patent for patent application no. 2021-513267 / registration no. 7489966 directed to Lantern Pharma’s drug candidate LP-284 ((+)N-hydroxy-N-(methylacylfulvene)urea). The Certificate of Patent entitled “Illudin Analogs, Uses Thereof, and Methods for Synthesizing the Same” covers molecule LP-284, including claims covering the new molecular entity. A Certificate of Patent is issued after JPO examinations have confirmed the merits of a patent request. Lantern values the broad protection this latest patent provides.
By Lantern Pharma Inc. · Via Business Wire · June 12, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, today announced operational highlights and financial results for the first quarter 2024, ended March 31, 2024.
By Lantern Pharma Inc. · Via Business Wire · May 9, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its first quarter 2024 financial results webcast on Thursday, May 9, at 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · May 2, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), a leading artificial intelligence (AI) oncology drug discovery and development company, today announced a strategic AI-driven collaboration with French biotechnology company, Oregon Therapeutics to optimize the development of its first-in-class protein disulfide isomerase (PDI)(1) inhibitor drug candidate XCE853 in novel and targeted cancer indications. Lantern will be leveraging its proprietary RADR® AI platform to uncover biomarkers and efficacy-associated signatures of XCE853 across solid tumors that can aid in precision development. Collaborative efforts are expected to identify biomarker signatures that can be used to stratify tumors most responsive to XCE853 and guide potential future clinical development and patient selection. Oregon Therapeutics is developing XCE853 in various cancer indications, including drug-resistant ovarian and pancreatic cancer, certain hematological cancers and several pediatric cancers including CNS cancers.
By Lantern Pharma Inc. · Via Business Wire · May 6, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, announced today that – the company has launched Webinar Wednesdays, a webinar series that focuses on areas of high oncology drug development interest with leading physicians, scientists and Lantern collaborators in drug development and artificial intelligence. The series begins today, Wednesday, April 24, 2024, and is planned to be held on the last Wednesday of each month.
By Lantern Pharma Inc. · Via Business Wire · April 24, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, announced today that – the company has received regulatory approval to expand its Harmonic™ trial, a Phase 2 clinical study evaluating LP-300 in non-small cell lung cancer (NSCLC) in never-smokers in both Japan and Taiwan. Approximately one third of all lung cancer patients in East Asia are never-smokers and the proportion of lung cancer in never smokers (LCINS) has been increasing gradually over time, according to a publication in Translational Lung Cancer Research (1).
By Lantern Pharma Inc. · Via Business Wire · April 22, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, today announced operational highlights and financial results for the fourth quarter and fiscal year ended December 31, 2023.
By Lantern Pharma Inc. · Via Business Wire · March 18, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted and transformative cancer therapies using its proprietary AI and machine learning (ML) platform, RADR®, with multiple clinical stage drug programs, today announced the dosing of the first two patients in the Phase 1 clinical trial evaluating Lantern’s investigational new drug LP-284 in patients with relapsed or refractory non-Hodgkin’s lymphoma (NHL), including mantle cell lymphoma (MCL) and double hit lymphoma (DHL) and other high-grade B-cell lymphomas (HGBL) as well as other select solid tumors and sarcomas. Recently, Lantern Pharma’s AI platform, RADR® is expected to exceed 100 billion data points during 2024, and has been crucial in uncovering and accelerating indications for LP-284 as well as other drug-candidates that are in development.
By Lantern Pharma Inc. · Via Business Wire · March 15, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its fourth quarter and fiscal year 2023 financial results webcast on Monday, March 18, 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · March 11, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), a leader in AI-driven cancer drug discovery and development, announced that it will be presenting at a virtual conference being hosted by HCW. The conference will be on Thursday, March 7th, and is a virtual event.
By Lantern Pharma Inc. · Via Business Wire · March 5, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), a leader in AI-driven cancer drug discovery and development, announced a series of important milestones related to the development, size, and advancement of RADR® -- its proprietary AI platform focused on transforming the cost, pace, and timeline of oncology drug development.
By Lantern Pharma Inc. · Via Business Wire · March 4, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), a leader in AI-driven cancer drug discovery and development, announced an important milestone in its antibody-drug conjugate (ADC) program. In collaboration with Bielefeld University, Lantern has generated a new class of highly specific and highly potent ADCs with a cryptophycin drug-payload.
By Lantern Pharma Inc. · Via Business Wire · February 15, 2024

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, today announced that the U.S. Food and Drug Administration (FDA) has granted LP-284 Orphan Drug Designation (ODD) for the treatment of high-grade B-cell lymphoma with MYC and BCL2 rearrangements.
By Lantern Pharma Inc. · Via Business Wire · November 30, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, today announced operational highlights and financial results for the third quarter ended September 30, 2023.
By Lantern Pharma Inc. · Via Business Wire · November 8, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its third quarter 2023 operating and financial results webcast on Wednesday, November 8, 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · November 1, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted and transformative cancer therapies using its proprietary AI and machine learning (ML) platform, RADR®, with multiple clinical stage drug programs, today announced that Lantern management will be presenting at the ThinkEquity Conference on Thursday, October 19, 2023, at the Mandarin Oriental in New York, NY.
By Lantern Pharma Inc. · Via Business Wire · October 10, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted and transformative cancer therapies using its proprietary AI and machine learning (ML) platform, RADR®, with multiple clinical stage drug programs, today announced the dosing of the first patient in the Phase 1 clinical trial evaluating Lantern’s investigational new drug LP-184 in patients with advanced solid tumors.
By Lantern Pharma Inc. · Via Business Wire · September 25, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted and transformative cancer therapies using its proprietary AI and machine learning (ML) platform, RADR®, with multiple clinical stage drug programs, today announced that the United States Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application for LP-284. LP-284 is being developed for the treatment of relapsed or refractory non-Hodgkin’s lymphoma (NHL), including mantle cell lymphoma (MCL) and double hit lymphoma (DHL) and other high-grade B-cell lymphomas (HGBL). Lantern expects to commence enrollment of patients for the first-in-human Phase 1 trial for LP-284 during the fourth quarter of 2023.
By Lantern Pharma Inc. · Via Business Wire · September 18, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted and transformative cancer therapies using its proprietary AI and machine learning (ML) platform, RADR®, with multiple clinical stage drug programs, today announced that it will present positive data highlighting the anti-tumor potency of its drug candidate LP-284 for non-Hodgkin’s lymphoma (NHL) at the Society of Hematologic Oncology (SOHO) Eleventh Annual Meeting occurring on Sept. 6 – 9, 2023, at the George R. Brown Convention Center in Houston, Texas.
By Lantern Pharma Inc. · Via Business Wire · August 31, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted and transformative cancer therapies using its proprietary AI and machine learning (ML) platform, RADR®, with multiple clinical stage drug programs, today announced a substantial increase in the power and capabilities of RADR® focused on improving the drug development process for immune checkpoint inhibitors (ICIs). These capabilities are expected to address the multiple challenges facing the increased usage of ICIs in cancer therapy. Since gaining regulatory approval in 2011, ICIs have improved the lives of tens of thousands of cancer patients as either monotherapies, and more recently, in combination regimens with other therapies. The success of ICIs has resulted in multiple competing ICI molecules, often from the same class, in overlapping cancer indications. Additionally, recent clinical trial failures reveal headwinds to the desired expansion of ICIs for a broader range of cancers and patient groups. Currently, there are over 5,200 ongoing clinical trials involving ICIs, many of these lacking adequate biomarker strategies or guidance from AI enabled approaches to optimize the selection of patient responder populations.
By Lantern Pharma Inc. · Via Business Wire · August 28, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (ML) platform with multiple clinical-stage drug programs, received a notice of allowance from the United States Patent and Trademark Office (USPTO) covering a method of treatment for Atypical Teratoid Rhabdoid Tumor (ATRT) using LP-184, an aggressive and rapidly growing form of cancer of the central nervous system (CNS).
By Lantern Pharma Inc. · Via Business Wire · August 14, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage drug programs, today announced operational highlights and financial results for the second quarter ended June 30, 2023.
By Lantern Pharma Inc. · Via Business Wire · August 9, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that it will host its second quarter 2023 operating and financial results webcast on Wednesday, August 9, 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · August 2, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that the Company will participate and present at four upcoming conferences:
By Lantern Pharma Inc. · Via Business Wire · July 17, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence ("AI") company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced the company has published new findings in Oncotarget demonstrating drug candidate LP-284’s in vitro and in vivo antitumor potency for multiple non-Hodgkin’s lymphomas (NHL), including mantle cell lymphoma (MCL) and double-hit lymphoma (DHL). The journal article titled “LP-284 Targets Non-Hodgkin's Lymphoma and DNA Damage Repair Deficiency” further supports LP-284’s development for NHL and advancement towards a first-in-human Phase 1 trial, which is anticipated for the second half of 2023.
By Lantern Pharma Inc. · Via Business Wire · June 26, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence ("AI") company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug programs, today announced that the U.S. Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application for LP-184, which is being developed for multiple advanced solid tumors and central nervous system (CNS) cancers. The first-in-human Phase 1A trial for LP-184 is anticipated to launch and dose its first patient during the current quarter.
By Lantern Pharma Inc. · Via Business Wire · June 12, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence ("AI") and machine learning (“ML”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced that Panna Sharma, CEO and President, will present at the Lytham Partners Spring 2023 Investor Conference on Thursday, May 18, 2023. The fireside chat-style webcast will be available to all registered attendees. Details of the webcast can be found below:
By Lantern Pharma Inc. · Via Business Wire · May 17, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence ("AI") and machine learning (“ML”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced operational highlights and financial results for the first quarter ended March 31, 2023.
By Lantern Pharma Inc. · Via Business Wire · May 9, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence (AI) and machine learning (ML) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced that it will host its first quarter 2023 operating and financial results webcast on Tuesday, May 9, 2023 at 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time.
By Lantern Pharma Inc. · Via Business Wire · May 2, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence ("AI") and machine learning (“ML”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced that it has developed highly-accurate and industry-leading AI algorithms to predict the ability of a drug or compound to pass the blood-brain-barrier (BBB), a highly selective border that can prevent drugs from entering brain tissues. The BBB prevents an estimated 98% of drugs from entering the brain, which presents a major hurdle for developing drugs to treat brain and central nervous system (CNS) cancers. Lantern’s AI BBB permeability prediction algorithms have a prediction accuracy of 89-92% and represent a rapid and cost-effective way to screen drugs or compounds to determine if they cross the BBB, which can accelerate the development of drug candidates for brain and CNS cancer patients.
By Lantern Pharma Inc. · Via Business Wire · May 2, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence ("AI") and machine learning (“ML”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced that the United States Patent and Trademark Office (USPTO) has issued a notice of allowance for U.S. patent application no. 17/192,838 directed to Lantern Pharma’s drug candidate LP-284 ((+)N-hydroxy-N-(methylacylfulvene)urea). The allowed application entitled “Illudin Analogs, Uses Thereof, and Methods for Synthesizing the same” covers the molecule LP-284, including claims covering the new molecular entity itself. A notice of allowance is issued after the USPTO determines that the prosecution on the merits of a patent has been completed and grants the patent upon payment of the patent issuance fee.
By Lantern Pharma Inc. · Via Business Wire · April 13, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence ("AI") and machine learning (“ML”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced the dosing of the first patient in the Phase 2 Harmonic™ clinical trial evaluating Lantern’s investigational new drug LP-300 in combination with chemotherapy for never smokers with advanced non-small cell lung cancer (NSCLC).
By Lantern Pharma Inc. · Via Business Wire · March 28, 2023

Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence ("AI") and machine learning (“ML”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced that Lantern management and employees will be presenting at three upcoming events:
By Lantern Pharma Inc. · Via Business Wire · March 21, 2023
