Articles from Genprex, Inc.

NEW YORK, Sept. 21, 2023 (GLOBE NEWSWIRE) -- via InvestorWire — Genprex Inc. (NASDAQ: GNPX) today announces its placement in an editorial published by NetworkNewsWire ("NNW"), one of 50+ trusted brands within the InvestorBrandNetwork (“IBN”), a multifaceted financial news and publishing company for private and public entities.
By Genprex, Inc. · Via GlobeNewswire · September 21, 2023

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced the opening for patient enrollment of its Acclaim-2 clinical trial. Acclaim-2 is an open-label, multi-center Phase 1/2 clinical trial evaluating the Company’s lead drug candidate, REQORSA™ Immunogene Therapy, in combination with Keytruda® (pembrolizumab) in patients with late-stage non-small cell lung cancer (NSCLC) whose disease progressed after treatment with Keytruda. In 2021, Genprex received U.S. Food and Drug Administration’s (FDA) Fast Track Designation for treatment of the Acclaim-1 patient population.
By Genprex, Inc. · Via Business Wire · March 31, 2022

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its President and Chief Executive Officer, Rodney Varner, will provide an overview of the Company’s gene therapies for cancer and diabetes to investors at the 2022 BIO Europe Spring Investor Conference.
By Genprex, Inc. · Via Business Wire · March 23, 2022

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its President and Chief Executive Officer, Rodney Varner, will provide an overview of the Company’s gene therapies for cancer and diabetes to investors at the 2022 BIO CEO and Investor Conference and at the 2022 Diamond Equity Research Conference.
By Genprex, Inc. · Via Business Wire · February 9, 2022

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its collaborators published positive preclinical data for the use of Genprex’s ONCOPREX® Nanoparticle Delivery System for delivery of a FAS DNA plasmid to treat metastatic colorectal cancer. Published in the journal Cancers1, the preclinical study found that tumor selective ONCOPREX nanoparticles carrying FAS DNA plasmids suppress human colon tumor growth in vivo in mouse models, indicating that this may be an effective therapy for human colorectal cancer.
By Genprex, Inc. · Via Business Wire · January 27, 2022

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced a plan to accelerate the opening of clinical trial sites for the Acclaim-1 clinical trial which combines the Company’s lead product candidate, REQORSA™ (quaratusugene ozeplasmid) Immunogene Therapy with AstraZeneca’s Tagrisso®, as a potential innovative treatment for non-small cell lung cancer (NSCLC). Under a collaboration agreement with a large network of integrated, community-based oncology practices, the opening of four clinical trial sites to enroll patients in the Phase 1 portion of Genprex’s Acclaim-1 clinical trial is underway, with an expectation of opening additional sites in the Phase 2 portion of the study.
By Genprex, Inc. · Via Business Wire · January 10, 2022

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced its participation in upcoming investor and healthcare conferences to be held in January 2022. Genprex’s President and Chief Executive Officer, Rodney Varner, and the Company’s Chief Medical Officer, Mark Berger, MD will lead the Company’s presentations.
By Genprex, Inc. · Via Business Wire · January 5, 2022
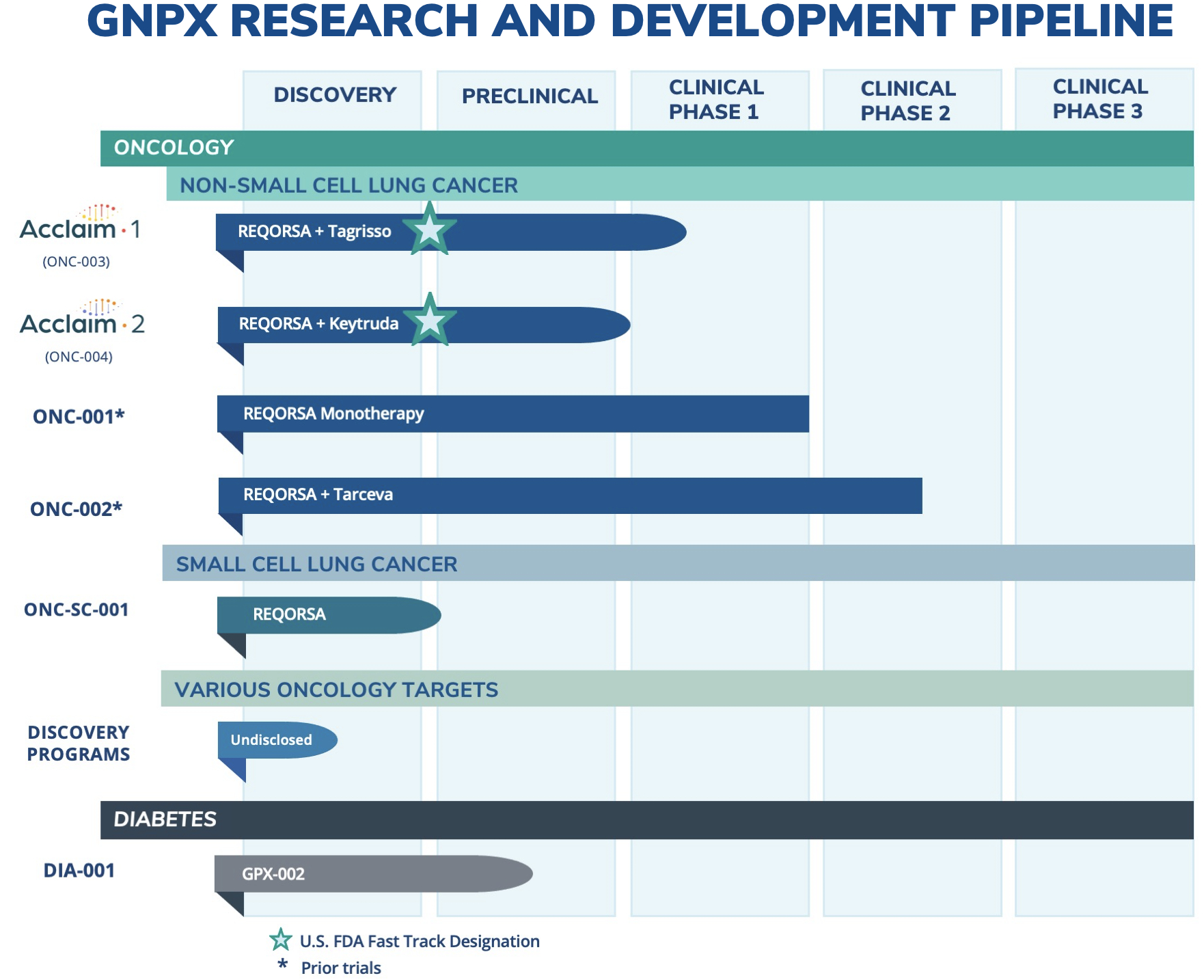
Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that it has expanded its oncology research and development pipeline to include small cell lung cancer (SCLC) as an additional disease indication for its lead drug candidate, REQORSA™ Immunogene Therapy. SCLC represents approximately 10-15 percent of the lung cancer market, while REQORSA’s initial target indication of non-small cell lung cancer (NSCLC) represents approximately 84 percent of the lung cancer market.
By Genprex, Inc. · Via Business Wire · January 4, 2022

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation (FTD) for the Company’s lead drug candidate, REQORSA™ Immunogene Therapy, in combination with Merck & Co’s Keytruda® in patients with histologically-confirmed unresectable stage III or IV non-small cell lung cancer (NSCLC) whose disease progressed after treatment with Keytruda. In the first quarter of 2022, Genprex expects to initiate its Acclaim-2 clinical trial, which is an open-label, multi-center Phase 1/2 clinical trial evaluating REQORSA in combination with Keytruda, for this patient population. The Company previously received its first FTD for REQORSA in combination with AstraZeneca PLC’s Tagrisso® in patients with histologically confirmed unresectable stage III or IV NSCLC, with EGFR mutations that progressed after treatment with Tagrisso.
By Genprex, Inc. · Via Business Wire · January 3, 2022

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its President and Chief Executive Officer, Rodney Varner, will be presenting in November with CEO Roadshow to provide a company overview of its novel gene therapies in cancer and diabetes to investors.
By Genprex, Inc. · Via Business Wire · November 16, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that the Company has strengthened its leadership team with the appointments of Mark S. Berger, M.D. to the newly-created position of Chief Medical Officer and Hemant Kumar, Ph.D., CPM, EMBA to the newly-created position of Chief Manufacturing and Technology Officer. Drs. Berger and Kumar will report to Rodney Varner, Chief Executive Officer of Genprex.
By Genprex, Inc. · Via Business Wire · September 28, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its President and Chief Executive Officer, Rodney Varner, will be participating in two investor conferences in September 2021.
By Genprex, Inc. · Via Business Wire · September 20, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its President and Chief Executive Officer, Rodney Varner, will be participating in a webinar series with CEO Roadshow to provide a company overview to investors on a monthly basis from July through September 2021.
By Genprex, Inc. · Via Business Wire · July 20, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that the U.S. Food and Drug Administration (FDA) has reviewed and confirmed all comments have been addressed regarding the Company’s clinical trial protocol for the Acclaim-1 clinical trial, an open-label, multi-center Phase 1/2 clinical trial evaluating the Company’s lead drug candidate, REQORSA™ Immunogene Therapy, in combination with AstraZeneca’s Tagrisso® in patients with late-stage non-small cell lung cancer (NSCLC) whose disease progressed after treatment with Tagrisso. In January 2020, Genprex received FDA Fast Track Designation for the Acclaim-1 patient population.
By Genprex, Inc. · Via Business Wire · June 23, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, announced that it will participate in Noble Capital Markets’ Virtual Roadshow Series, presented by Channelchek on May 20, 2021.
By Genprex, Inc. · Via Business Wire · May 19, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, announced that it will participate in the following investor conferences in the month of May, with presentations led by the Company’s President and Chief Executive Officer, Rodney Varner.
By Genprex, Inc. · Via Business Wire · May 10, 2021

Genprex, Inc. (“Genprex” or the “Company”) (Nasdaq: GNPX), a clinical-stage gene therapy company focused on developing life-changing technologies for patients with cancer and diabetes, today announced that the Company and a major cancer research center in Houston, Texas, in March 2021, entered into an amendment (the “Amendment”) to their May 2020 License Agreement (the “License Agreement”) to grant to Genprex an exclusive worldwide license to an additional portfolio of six patents and one patent application and related technology (“Newly Licensed IP”). The Newly Licensed IP includes methods for treating non-small cell lung cancer (NSCLC) by administration of a TUSC2 therapeutic in conjunction with EGFR inhibitors or other anti-cancer therapies, in patients who are predicted to be responsive to TUSC2 therapy. A TUSC2 gene-expressing plasmid is the active agent in REQORSA™ immunogene therapy, Genprex’s lead drug candidate.
By Genprex, Inc. · Via Business Wire · May 6, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that the Company has received centralized Institutional Review Board (IRB) approval of the clinical trial protocol for its upcoming Acclaim-1 clinical trial in non-small cell lung cancer (NSCLC). Acclaim-1 is an open-label, multi-center Phase 1/2 clinical trial that combines the Company’s lead drug candidate, REQORSA™ immunogene therapy, with AstraZeneca’s Tagrisso® (osimertinib) in patients with late-stage NSCLC with mutated epidermal growth factor receptors (EGFRs), whose disease progressed after treatment with Tagrisso.
By Genprex, Inc. · Via Business Wire · May 5, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, is pleased to announce it has commenced clinical trial site recruitment for its upcoming Acclaim-2 clinical trial for the treatment of non-small cell lung cancer (NSCLC).
By Genprex, Inc. · Via Business Wire · May 4, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ:GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its President and Chief Executive Officer, Rodney Varner, will be participating in a webinar series with CEO Roadshow to provide a company overview to investors on a weekly basis from April 22 through June 10, 2021.
By Genprex, Inc. · Via Business Wire · April 21, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, announces today that the Company has been selected to receive the inaugural “License of the Year” award from the University of Pittsburgh Innovation Institute (UPII) in recognition of the advances made with its license from University of Pittsburgh toward progressing the development of its gene therapy for diabetes.
By Genprex, Inc. · Via Business Wire · April 20, 2021

Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its collaborators presented positive preclinical data for the combination of TUSC2 immunogene therapy (REQORSA™) in combination with chemotherapy and immunotherapies for the treatment of non-small cell lung cancer (NSCLC). Collaborators also presented positive preclinical data for the use of REQORSA in combination with targeted therapies for the treatment of NSCLC. These data were presented in two presentations at the 2021 American Association of Cancer Research (AACR) annual meeting. The TUSC2 gene is a tumor suppressor gene and is the active agent in REQORSA.
By Genprex, Inc. · Via Business Wire · April 12, 2021
