Articles from Edgewise Therapeutics

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the company will present data on sevasemten, an investigational orally administered first-in-class fast skeletal myosin inhibitor designed to protect against contraction-induced muscle damage, at the Muscular Dystrophy Association (MDA) Clinical and Scientific Conference. The conference will take place at the Hilton Anatole, Dallas, TX from March 16-19, 2025.
By Edgewise Therapeutics · Via Business Wire · March 11, 2025

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that management will present at the Leerink Partners Global Healthcare Conference on Tuesday, March 11, 2025, at 1:40 pm ET.
By Edgewise Therapeutics · Via Business Wire · March 4, 2025

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced key changes to its executive team that will strengthen its leadership and sharpen the Company’s focus on late-stage clinical development. These changes include the addition of Robert Blaustein, M.D., Ph.D., joining the Company as Chief Development Officer and the promotion of Behrad Derakhshan, Ph.D. from Chief Business Officer to Chief Operating Officer.
By Edgewise Therapeutics · Via Business Wire · January 22, 2025

Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today provided recent corporate updates and highlighted upcoming priorities for 2025. Edgewise Chief Executive Officer, Kevin Koch, Ph.D., will present these updates today at the Annual J.P. Morgan Healthcare Conference.
By Edgewise Therapeutics · Via Business Wire · January 13, 2025

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that Kevin Koch, Ph.D, President and Chief Executive Officer, will present at the 43rd Annual J.P. Morgan Healthcare Conference on Monday, January 13, 2025, at 1:30 pm PT (4:30 pm ET). The discussion will include updates on its cardiovascular and muscular dystrophy programs and 2025 key milestones.
By Edgewise Therapeutics · Via Business Wire · January 7, 2025

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced positive topline results from the Phase 2 CANYON trial of sevasemten in individuals with Becker muscular dystrophy. Sevasemten is an orally administered first-in-class fast skeletal myosin inhibitor designed to protect muscle against contraction-induced damage in muscular dystrophies. The trial met its primary endpoint of change from baseline in CK. CANYON is the largest interventional trial to date in Becker and the first to achieve its primary endpoint.
By Edgewise Therapeutics · Via Business Wire · December 16, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today provided information regarding the company’s relationship with Dr. Han Phan at Rare Disease Research.
By Edgewise Therapeutics · Via Business Wire · December 5, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that management will present at the Piper Sandler 36th Annual Healthcare Conference on Tuesday, December 3, 2024, at 1 pm ET.
By Edgewise Therapeutics · Via Business Wire · November 26, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today reported financial results for the third quarter of 2024 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · November 7, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced its participation at the 29th International Annual Congress of the World Muscle Society (WMS) with an industry-sponsored symposium and the presentation of seven scientific posters. These presentations will highlight the effects of sevasemten in individuals with Becker based on findings from the DUNE and ARCH clinical trials, as well as provide perspectives on biomarker and functional endpoints being studied in the CANYON Phase 2 trial. Sevasemten is an orally administered small molecule designed to prevent contraction-induced muscle damage in muscular dystrophies including Becker and Duchenne muscular dystrophy. The conference will take place at The Prague Congress Centre in Prague, Czechia, October 8-12, 2024.
By Edgewise Therapeutics · Via Business Wire · October 1, 2024
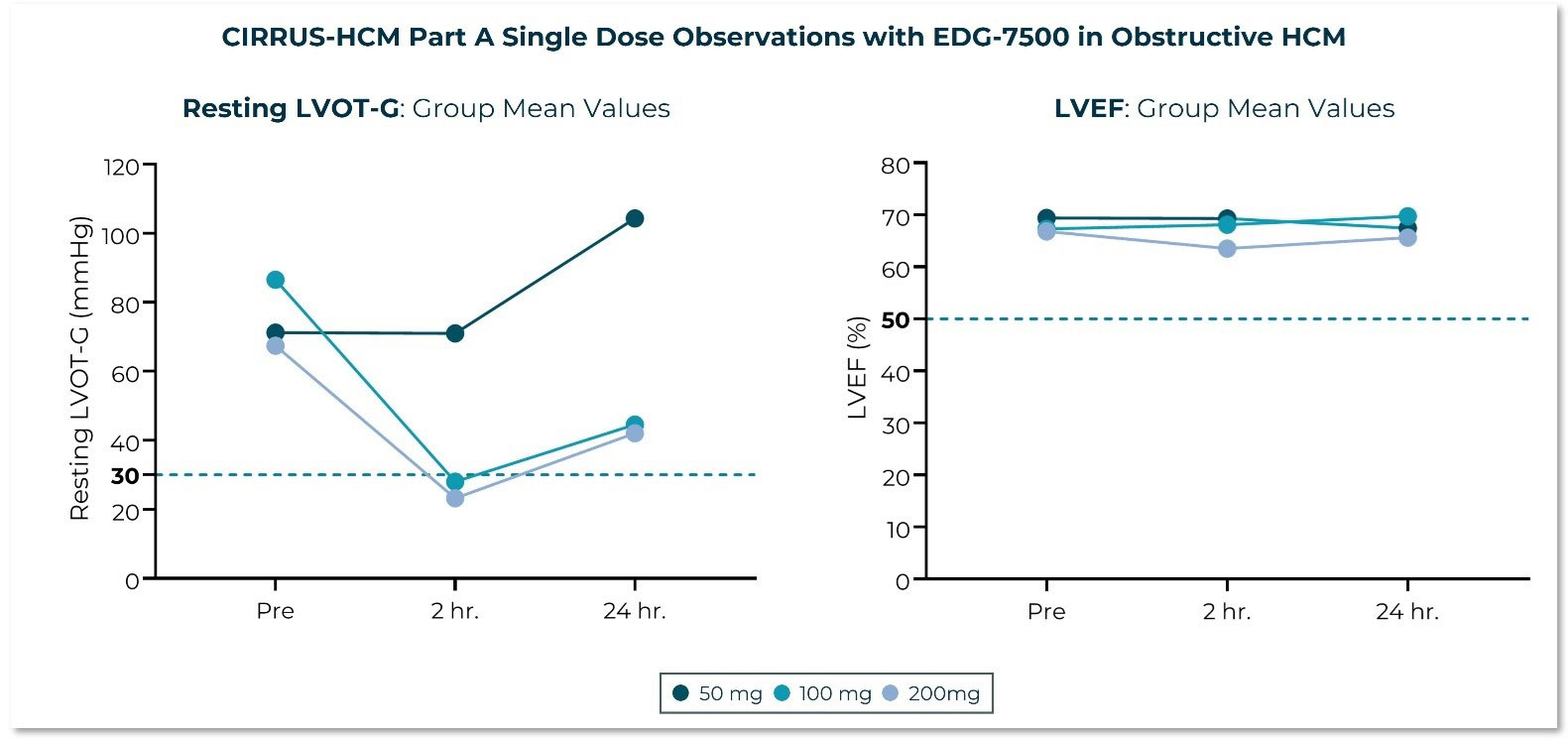
Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced top-line data of EDG-7500 from the Phase 1 trial in healthy subjects and the single-dose arm of the Phase 2 CIRRUS-HCM trial in patients with obstructive HCM. EDG-7500 is a novel oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM.
By Edgewise Therapeutics · Via Business Wire · September 19, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that members of the management team will hold a live webcast to discuss top-line data of EDG-7500 from the Phase 1 trial in healthy subjects and the single-dose arm of the Phase 2 CIRRUS-HCM trial in patients with obstructive HCM on Thursday, September 19, 2024, at 8:30 am ET. The team will be joined by CIRRUS-HCM investigator, Anjali T. Owens, M.D., Medical Director, Center for Inherited Cardiac Disease, Associate Professor of Medicine, University of Pennsylvania, who will share her perspective of EDG-7500 and HCM. An accompanying slide presentation will also be available. To register for the live webcast and replay, please visit the Edgewise events page.
By Edgewise Therapeutics · Via Business Wire · September 17, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today reported financial results for the second quarter of 2024 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · August 8, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that management will present at the RBC Capital Markets Global Healthcare Conference on Wednesday, May 15, 2024, at 3:35 pm ET.
By Edgewise Therapeutics · Via Business Wire · May 10, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today reported financial results for the first quarter of 2024 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · May 9, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced the appointment of biotechnology industry veteran Arlene Morris to its Board of Directors. Ms. Morris has extensive experience in the pharmaceutical and biotechnology industries serving in numerous executive management and board roles.
By Edgewise Therapeutics · Via Business Wire · May 7, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced the dosing of the first patient in the Phase 2 CIRRUS-HCM trial of EDG-7500. EDG-7500 is a novel oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM and other diseases of diastolic dysfunction. The Phase 2 trial will assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics of EDG-7500 in patients with obstructive HCM. Part A of the trial will evaluate single doses and Part B will evaluate multiple oral doses of EDG-7500 over 28 days.
By Edgewise Therapeutics · Via Business Wire · May 6, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the European Medicines Agency (EMA) has granted Orphan Drug Designations for sevasemten for the treatment of Becker muscular dystrophy (Becker) and for the treatment of Duchenne muscular dystrophy (Duchenne). Sevasemten is an investigational orally administered small molecule designed to prevent contraction-induced muscle damage. Sevasemten is currently in late-stage clinical trials for individuals with Becker and is also being studied in Duchenne.
By Edgewise Therapeutics · Via Business Wire · April 23, 2024
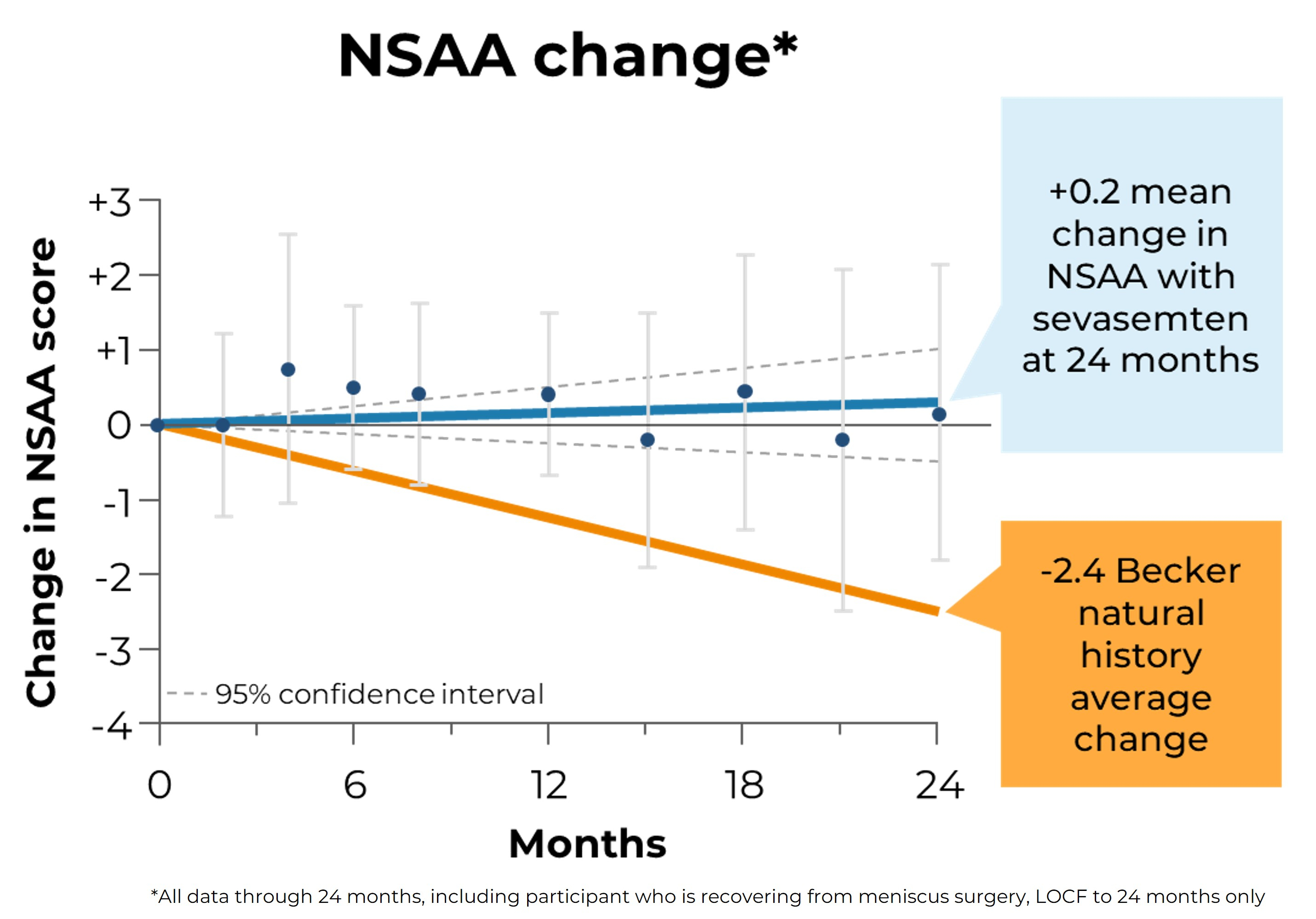
Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced positive two-year topline results from the ARCH trial. ARCH is an open label, single-center study assessing safety, tolerability, impact on muscle damage biomarkers, pharmacokinetics (PK) and functional measures with sevasemten (EDG-5506) in adults with Becker. Sevasemten is an orally administered small molecule designed to prevent contraction-induced muscle damage in dystrophinopathies including Becker and Duchenne muscular dystrophy (Duchenne).
By Edgewise Therapeutics · Via Business Wire · April 15, 2024

Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the Company will present data on EDG-7500 at the American College of Cardiology's Annual Scientific Session (ACC.24). EDG-7500 is a first-in-class oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM and other diseases of diastolic dysfunction. The conference will take place in Atlanta, GA at the Georgia World Congress Center from April 6-8, 2024.
By Edgewise Therapeutics · Via Business Wire · March 28, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that management will present at the Cantor Virtual Muscular Dystrophy Symposium on Tuesday, April 2, 2024, at 2:20 pm ET.
By Edgewise Therapeutics · Via Business Wire · March 27, 2024

Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that management will present at the Leerink Partners Global Biopharma Conference on Monday, March 11, 2024, at 12:00 pm ET.
By Edgewise Therapeutics · Via Business Wire · March 5, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the company will present data on EDG-5506, an investigational therapy designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies, at the Muscular Dystrophy Association (MDA) Clinical and Scientific Conference. The conference will take place at the Hilton Orlando in Orlando, FL from March 3-6, 2024.
By Edgewise Therapeutics · Via Business Wire · February 28, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today reported financial results for the fourth quarter and full year of 2023 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · February 22, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for EDG-5506 for the treatment of Duchenne. EDG-5506 is an investigational orally administered small molecule designed to prevent contraction-induced muscle damage in dystrophinopathies, including Duchenne and Becker muscular dystrophy (Becker). The FDA previously granted EDG-5506 Orphan Drug Designation (ODD) for the treatment of Duchenne and Becker, Rare Pediatric Disease Designation (RPDD) for the treatment of Duchenne, and Fast Track designation for the treatment of Becker.
By Edgewise Therapeutics · Via Business Wire · February 13, 2024

Edgewise Therapeutics, Inc. (NASDAQ: EWTX), a leading muscle disease biopharmaceutical company, today announced the pricing of an underwritten offering of 21,818,182 shares of its common stock at an offering price of $11.00 per share. Edgewise anticipates gross proceeds from the offering to be approximately $240 million, before deducting underwriting discounts and commissions and offering expenses. The closing of the offering is expected to occur on January 23, 2024, subject to the satisfaction of customary closing conditions.
By Edgewise Therapeutics · Via Business Wire · January 19, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, will present today at the 42nd Annual J.P. Morgan Healthcare Conference at 10:30 am PT (1:30 pm ET), and a live webcast will be available at www.edgewisetx.com. Ahead of the presentation, the Company highlighted its 2023 accomplishments and announced its anticipated key milestones for 2024.
By Edgewise Therapeutics · Via Business Wire · January 9, 2024

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that management will present at the J.P. Morgan 42nd Annual Healthcare Conference on Tuesday, January 9, 2024, at 10:30 am PT (1:30 pm ET).
By Edgewise Therapeutics · Via Business Wire · December 19, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced the launch of an educational website dedicated to the Becker muscular dystrophy (Becker) community: www.beckermusculardystrophy.com. This is the first website solely focused on providing Becker-specific resources to help individuals and caregivers better understand the disease, learn different approaches to care and stay up to date on advocacy partnerships and available services.
By Edgewise Therapeutics · Via Business Wire · December 18, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the U.S. Food & Drug Administration (FDA) has granted EDG-5506 Orphan Drug Designation (ODD) for the treatment of Duchenne muscular dystrophy (Duchenne) and Becker muscular dystrophy (Becker) and Rare Pediatric Disease Designation (RPDD) for the treatment of Duchenne. EDG-5506 is an investigational orally administered small molecule designed to prevent contraction-induced muscle damage in dystrophinopathies, including Duchenne and Becker. EDG-5506 is currently advancing in multiple Phase 2 trials for individuals with Duchenne, Becker and other dystrophinopathies. The FDA previously granted Fast Track designation for the investigation and development of EDG-5506 for the treatment of Becker.
By Edgewise Therapeutics · Via Business Wire · November 30, 2023

Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today reported financial results for the third quarter of 2023 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · November 9, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, announced today that members of its senior management team will participate in the following upcoming investor conferences:
By Edgewise Therapeutics · Via Business Wire · November 8, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the Company will present the results of nonclinical studies of EDG-7500 at the American Heart Association’s (AHA) Scientific Sessions 2023 being held in Philadelphia from November 11 - 13. EDG-7500 is a first-in-class oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with hypertrophic cardiomyopathy (HCM) and other diseases of diastolic dysfunction.
By Edgewise Therapeutics · Via Business Wire · November 1, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced an expansion of their clinical development program of EDG-5506, an investigational orally administered small molecule designed to prevent contraction-induced muscle damage in dystrophinopathies, including Duchenne. The Company is initiating FOX, a new Phase 2 placebo-controlled trial in children and adolescent boys with Duchenne who have been previously treated with gene therapy. Further, the Company is continuing dose escalation and expanding enrollment in their Phase 2 placebo-controlled LYNX trial; one of the new LYNX cohorts will study EDG-5506 in boys with Duchenne not currently treated with corticosteroids.
By Edgewise Therapeutics · Via Business Wire · October 26, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the Company will present on EDG-5506, an orally administered small molecule designed to prevent contraction-induced muscle damage in dystrophinopathies, at the 28th International Annual Congress of the World Muscle Society (WMS). The conference will take place at the Charleston Convention Center in Charleston, South Carolina, from October 3-7, 2023.
By Edgewise Therapeutics · Via Business Wire · September 27, 2023

Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced the start of enrollment of GRAND CANYON, a global pivotal study of EDG-5506 in individuals with Becker. GRAND CANYON is an expansion of the CANYON study. CANYON, which was over-enrolled, includes 39 adults and 24 adolescents. EDG-5506 is an orally administered small molecule designed to prevent contraction-induced muscle damage in dystrophinopathies including Becker and Duchenne muscular dystrophy. There are currently no approved therapies for individuals with Becker, a serious genetic, progressive neuromuscular disorder with significant unmet need.
By Edgewise Therapeutics · Via Business Wire · September 26, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced initial dosing in a Phase 1 trial of EDG-7500. EDG-7500 is a first-in-class oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM and other diseases of diastolic dysfunction. The Phase 1 trial will assess the safety, tolerability, pharmacokinetics and pharmacodynamics of EDG-7500 in healthy adults. The Company is also planning to begin a Phase 1b study of EDG-7500 in individuals with obstructive HCM in the first half of 2024.
By Edgewise Therapeutics · Via Business Wire · September 14, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a clinical-stage biopharmaceutical company focused on the discovery, development, and commercialization of innovative treatments for severe, genetic neuromuscular and cardiac disorders for which there is significant unmet medical need, today reported financial results for the second quarter of 2023 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · August 10, 2023
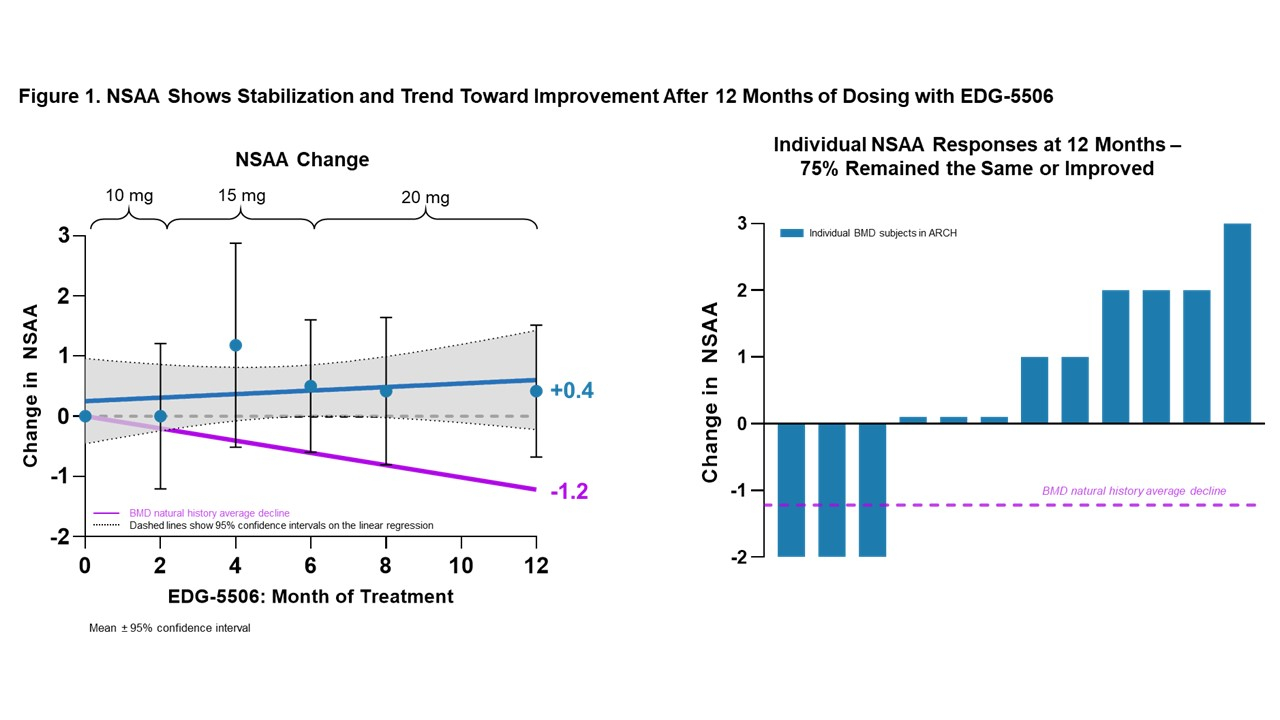
Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, announced today positive 12-month topline results from the ongoing ARCH study, an open label, single-center study assessing the safety, tolerability, impact on muscle damage biomarkers, and pharmacokinetics (PK) of EDG-5506 in adults with BMD. EDG-5506 is an orally administered small molecule designed to prevent contraction-induced muscle damage in dystrophinopathies including BMD and Duchenne muscular dystrophy (DMD).
By Edgewise Therapeutics · Via Business Wire · June 26, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, announced today that management will present at the Goldman Sachs Global Healthcare Conference on Tuesday, June 13, 2023, at 10 am PT (1 pm ET).
By Edgewise Therapeutics · Via Business Wire · June 6, 2023

Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today reported financial results for the first quarter of 2023 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · May 11, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, announced today that management will participate in fireside chats at two upcoming investor conferences:
By Edgewise Therapeutics · Via Business Wire · May 2, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today announced the publication of the article, “Modulating fast skeletal muscle contraction protects skeletal muscle in animal models of Duchenne muscular dystrophy,” in the Journal of Clinical Investigation.
By Edgewise Therapeutics · Via Business Wire · March 30, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today announced that management will present at the virtual Stifel 2023 CNS Days on Tuesday, March 28, 2023, at 2 pm ET.
By Edgewise Therapeutics · Via Business Wire · March 21, 2023

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today announced that the company will present on EDG-5506, an investigational therapy designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies, at the Muscular Dystrophy Association (MDA) Annual Clinical and Scientific Conference. The conference will take place at the Hilton Anatole in Dallas, TX from March 19-22, 2023.
By Edgewise Therapeutics · Via Business Wire · March 14, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for individuals with devastating muscle disorders, announced today the appointment of Jonathan C. Fox, M.D., Ph.D., FACC, to its Board of Directors. Dr. Fox brings considerable expertise in clinical development and regulatory strategy to Edgewise, serving currently as President and CMO at BridgeBio Pharma, and previously as CMO at MyoKardia, where he was one of the inventors of Camzyos® (mavacamten) for the treatment of obstructive hypertrophic cardiomyopathy (oHCM).
By Edgewise Therapeutics · Via Business Wire · March 6, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today announced that management will present at the Cowen 43rd Annual Health Care Conference on Wednesday, March 8, 2023, at 9:10 am ET.
By Edgewise Therapeutics · Via Business Wire · March 1, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today announced that the company will present on EDG-7500, an investigational therapy designed to normalize excess cardiac contraction (systolic) and deficient relaxation (diastolic), the underlying pathology of both obstructive and non-obstructive HCM, at the American College of Cardiology's 72nd Annual Scientific Session Together with World Congress of Cardiology (ACC.23/WCC). The conference will take place at the New Orleans Ernest N. Morial Convention Center from March 4-6, 2023.
By Edgewise Therapeutics · Via Business Wire · February 28, 2023

Edgewise Therapeutics, Inc. (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today reported financial results for the fourth quarter and full year of 2022 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · February 23, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today announced that management will present at the virtual SVB Securities Global Biopharma Conference on Tuesday, February 14, 2023, at 1:00 pm ET.
By Edgewise Therapeutics · Via Business Wire · February 7, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, today announced that management will present at the J.P. Morgan 41st Annual Healthcare Conference on Tuesday, January 10, 2023, at 9:45 am PT (12:45 pm ET).
By Edgewise Therapeutics · Via Business Wire · January 4, 2023

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for the treatment of devastating muscle disorders, announced today plans to host a virtual investor call on Tuesday, January 3, 2023, at 8:30 am ET. The webcast event will feature a presentation of preclinical results for its lead cardiac program candidate, EDG-7500, initially targeting patients with HCM and will include commentary by a leading cardiology expert.
By Edgewise Therapeutics · Via Business Wire · December 21, 2022

Edgewise Therapeutics, Inc. (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, targeted, small molecule therapies for individuals with devastating muscle disorders, announced today the appointment of Marc Semigran, M.D., as Chief Development Officer. Dr. Semigran brings considerable clinical development and translational medicine experience to Edgewise, having most recently served as Chief Medical Officer (CMO) at Renovacor through its acquisition by Rocket Pharmaceuticals, Inc., and previously served as CMO and Senior Vice President of medical science at MyoKardia through its acquisition by Bristol Myers Squibb in 2020 for $13.1 billion. He will be responsible for leading cardiovascular development at Edgewise.
By Edgewise Therapeutics · Via Business Wire · December 20, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today that members of its senior management team will participate in the following upcoming investor conferences:
By Edgewise Therapeutics · Via Business Wire · November 9, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today reported financial results for the third quarter of 2022 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · November 3, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today announced that it has initiated the LYNX Phase 2 clinical trial at Rare Disease Research, LLC in Atlanta, Georgia. The trial will examine the safety, pharmacokinetics (PK) and effect on biomarkers of muscle damage of EDG-5506 in children with DMD. The study will also explore changes in functional measures, such as the North Star Ambulatory Assessment (NSAA) and self-reported/caregiver-reported outcomes. EDG-5506 is an investigational orally administered small molecule myosin modulator designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies such as DMD and Becker muscular dystrophy (BMD).
By Edgewise Therapeutics · Via Business Wire · October 27, 2022
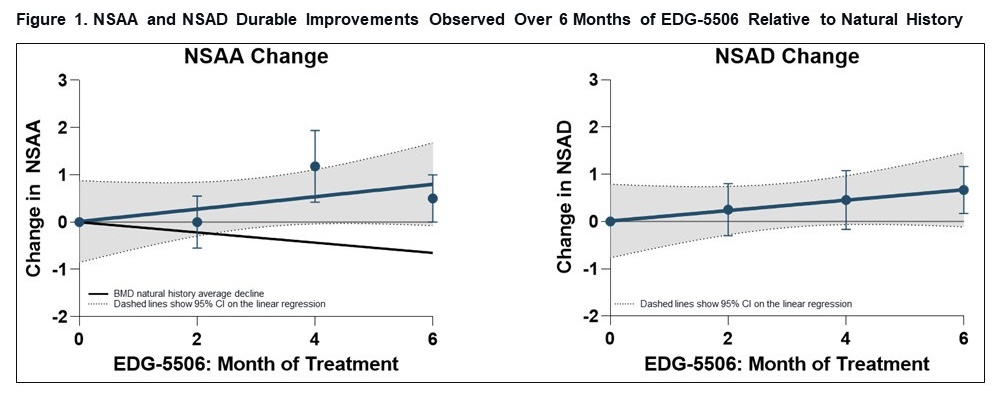
Edgewise Therapeutics, Inc. (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today positive 6-month interim results from the ongoing ARCH study, an open label, single-center study assessing the safety, tolerability, impact on muscle damage biomarkers, and pharmacokinetics (PK) of EDG-5506 in adults with BMD. EDG-5506 is an investigational orally administered small molecule myosin modulator designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies such as Duchenne muscular dystrophy (DMD) and BMD.
By Edgewise Therapeutics · Via Business Wire · October 13, 2022

Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today announced that the Company will present on EDG-5506, an investigational therapy designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies, at the 27th International Hybrid Annual Congress of the World Muscle Society (WMS). The conference will take place at the Halifax Convention Centre in Halifax, Nova Scotia, from October 11-15, 2022.
By Edgewise Therapeutics · Via Business Wire · October 6, 2022

Edgewise Therapeutics, Inc. (NASDAQ: EWTX) today announced the pricing of its upsized underwritten public offering of 11,627,907 shares of its common stock at a price to the public of $10.32 per share. All of the shares are to be sold by Edgewise Therapeutics. In addition, Edgewise Therapeutics has granted the underwriters a 30-day option to purchase up to an additional 1,744,186 shares of its common stock. Before deducting the underwriting discounts and commissions and offering expenses, Edgewise Therapeutics expects to receive total gross proceeds of $120 million, assuming no exercise of the underwriters’ option to purchase additional shares. The offering is expected to close on or about September 16, 2022, subject to satisfaction of customary closing conditions.
By Edgewise Therapeutics · Via Business Wire · September 13, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX) today announced that it intends to offer and sell shares of its common stock in an underwritten public offering of $100 million of its common stock. In addition, Edgewise Therapeutics intends to grant the underwriters a 30-day option to purchase up to an additional $15.0 million of its common stock at the public offering price, less underwriting discounts and commissions. All of the shares of common stock in this offering will be sold by Edgewise. The proposed offering is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed, or as to the actual size or terms of the offering.
By Edgewise Therapeutics · Via Business Wire · September 13, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today positive 4-month interim results from the ongoing ARCH study, an open label, single-center study assessing the safety, tolerability, impact on muscle damage biomarkers, and pharmacokinetics (PK) of EDG-5506 in adults with BMD. EDG-5506 is an investigational orally administered small molecule myosin modulator designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies such as Duchenne muscular dystrophy (DMD) and BMD.
By Edgewise Therapeutics · Via Business Wire · September 11, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today announced that the U.S. Food and Drug Administration (FDA) has authorized a clinical trial of EDG-5506 in children with DMD. The Company expects to begin dosing participants in the LYNX Phase 2 trial in the fourth quarter of 2022. EDG-5506 is an investigational orally administered small molecule myosin modulator designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies such as DMD and Becker muscular dystrophy (BMD).
By Edgewise Therapeutics · Via Business Wire · September 7, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today that members of its senior management team will participate in a panel discussion and one-on-one investor meetings at the virtual 2022 Wedbush PacGrow Healthcare Conference on August 9 and 10.
By Edgewise Therapeutics · Via Business Wire · August 3, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today announced the initiation of the CANYON Phase 2 clinical trial evaluating EDG-5506 in individuals with BMD. EDG-5506 is an orally administered small molecule myosin modulator designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies such as BMD and Duchenne muscular dystrophy (DMD). There are currently no approved treatments for BMD.
By Edgewise Therapeutics · Via Business Wire · July 12, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today positive 2-month interim results from the ongoing ARCH study, an open label, single-center study assessing the safety, tolerability, impact on muscle damage biomarkers, and pharmacokinetics (PK) of EDG-5506 in adults with BMD. EDG-5506 is an orally administered small molecule myosin inhibitor designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies such as Duchenne muscular dystrophy (DMD) and BMD.
By Edgewise Therapeutics · Via Business Wire · June 20, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for rare muscle disorders, today announced that new 2-month interim data from the ARCH open label study of EDG-5506 in individuals with BMD will be presented at the 2022 New Directions in Biology and Disease of Skeletal Muscle Conference, being held June 20-23, 2022.
By Edgewise Therapeutics · Via Business Wire · June 16, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today that members of its senior management team will participate in the following upcoming investor conferences:
By Edgewise Therapeutics · Via Business Wire · May 31, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today reported financial results for the first quarter of 2022 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · May 11, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today that members of its senior management team will participate in fireside discussions and one-on-one investor meetings at two upcoming investor conferences:
By Edgewise Therapeutics · Via Business Wire · May 3, 2022

Edgewise Therapeutics, Inc. (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today announced the initiation of an Edgewise-funded observational trial in individuals with BMD. The trial is designed to understand the disease progression of individuals with BMD as assessed by functional measures and imaging endpoints. This global, multi-center trial is led by the GRASP (General Resolution and Assessments Solving Phenotypes) consortium and Virginia Commonwealth University (VCU), in collaboration with ImagingDMD University of Florida (UF).
By Edgewise Therapeutics · Via Business Wire · April 14, 2022

Edgewise Therapeutics, Inc. (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today reported financial results for the fourth quarter and full year of 2021 and recent business highlights.
By Edgewise Therapeutics · Via Business Wire · February 24, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today that members of its senior management team will participate in a fireside discussion and one-on-one investor meetings at the SVB Leerink Virtual 11th Annual Global Healthcare Conference, on February 16, 2022.
By Edgewise Therapeutics · Via Business Wire · February 2, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today positive topline results from the BMD, or Phase 1b, portion of a first-in-human Phase 1 clinical trial assessing the safety, tolerability, PK and pharmacodynamics (PD) of EDG-5506, an orally administered small molecule myosin inhibitor designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies such as Duchenne muscular dystrophy (DMD) and BMD.
By Edgewise Therapeutics · Via Business Wire · January 5, 2022

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, today announced plans to report topline results from the Phase 1b clinical trial of EDG-5506 in individuals with BMD on January 5, 2022, at 9:00 am ET. The webcast event will feature a presentation of the results which support advancement of the program, and commentary by a leading neuromuscular disease expert. Additionally, the Company will provide details on the recently initiated follow-on open label study, EDG-5506-002 (ARCH).
By Edgewise Therapeutics · Via Business Wire · December 15, 2021

Edgewise Therapeutics, Inc., (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today that members of its senior management team will participate in a fireside chat and one-on-one investor meetings at the virtual Evercore ISI 4th Annual HEALTHCONx conference on December 2.
By Edgewise Therapeutics · Via Business Wire · November 17, 2021
