Articles from Dr. Reddy’s Laboratories Ltd.

Dr. Reddy’s Laboratories Ltd. (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY) today announced its consolidated financial results for the quarter and nine months ended December 31, 2024. The information mentioned in this release is based on consolidated financial statements under International Financial Reporting Standards (IFRS).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · January 23, 2025

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; along with its subsidiaries together referred to as “Dr. Reddy’s”), announced the launch of Toripalimab in India.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · November 28, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY) today announced its consolidated financial results for the quarter and half year ended September 30, 2024. The information mentioned in this release is based on consolidated financial statements under International Financial Reporting Standards (IFRS).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · November 5, 2024

Dr. Reddy’s Laboratories Ltd. (“Dr. Reddy’s”) has been ranked among the top employers according to the 2024 Top Biotech and Pharma Employers Survey conducted by Science.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · October 25, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; along with its subsidiaries together referred to as “Dr. Reddy’s”), today announced that it has entered into a royalty-free non-exclusive voluntary licensing agreement with Gilead Sciences Ireland UC for the manufacture and commercialisation of the drug, Lenacapavir, in India and 120 other countries.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · October 2, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; along with its subsidiaries together referred to as “Dr. Reddy’s”), and its CRDMO arm Aurigene Pharmaceutical Services Limited (Aurigene), today announced that they have signed a non-binding Memorandum of Understanding (MoU) with Kainomyx, Inc., a US-based company with a proprietary platform that helps target cytoskeletal proteins of parasites, a novel mechanism of action, for the development and commercialization of affordable anti-malarial drug in the U.S., Europe, and in low and middle-income countries. The MoU remains subject to signing of a definitive agreement.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · August 20, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; along with its subsidiaries together referred to as “Dr. Reddy’s”), today announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending the launch of its proposed biosimilar Rituximab candidate DRL_RI (ITUXREDI®) in European markets.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · July 29, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY) today announced its consolidated financial results for the quarter ended June 30, 2024. The information mentioned in this release is based on consolidated financial statements under International Financial Reporting Standards (IFRS).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · July 27, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; along with its subsidiaries together referred to as “Dr. Reddy’s”), a global pharmaceutical company, today announced that its subsidiary Dr. Reddy's Laboratories SA has signed a definitive agreement with Haleon plc (LSE/NYSE: HLN; “Haleon”), a leading consumer healthcare company, for purchase of shares of Northstar Switzerland SARL, a Haleon group company, to acquire Haleon’s global portfolio of consumer healthcare brands in the Nicotine Replacement Therapy (“NRT”) category outside of the United States. The portfolio to be acquired consists of Nicotinell, a global leader in the NRT category with an extensive footprint in over 30 countries spanning Europe, Asia including Japan, and Latin America, and local market-leading brand names of the product – Nicabate in Australia, Thrive in Canada, and Habitrol in New Zealand and Canada. The proposed acquisition will be inclusive of all formats such as lozenge, patch, gum as well as pipeline products, in all applicable global markets outside of the United States.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · June 26, 2024

Alvotech (NASDAQALVO), a global biotech company specializing in the development and manufacture of biosimilar medicines for patients worldwide (“Alvotech”), and Dr. Reddy’s Laboratories SA, wholly-owned subsidiary of Dr. Reddy’s Laboratories Ltd., (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY, along with its subsidiaries hereafter referred to as “Dr. Reddy’s”), today announced that the companies have entered into a license and supply agreement for the commercialization of AVT03, Alvotech’s biosimilar candidate to Prolia® and Xgeva® (denosumab). The collaboration combines Dr. Reddy’s global commercial presence with Alvotech’s proven capabilities in developing biosimilars for markets worldwide.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · May 21, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY) today announced its consolidated financial results for the fourth quarter and full year ended March 31, 2024. The information mentioned in this release is based on consolidated financial statements under International Financial Reporting Standards (IFRS).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · May 7, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; hereafter referred to as “Dr. Reddy’s”), a global pharmaceutical company, announced the launch of Versavo® (bevacizumab) in the United Kingdom (UK). Dr. Reddy’s Versavo® is a (bevacizumab) biosimilar of Avastin®1 and indicated for the treatment of several types of cancers, including metastatic colorectal cancer, advanced non-squamous non-small cell lung cancer, recurrent glioblastoma, metastatic renal cell carcinoma, advanced cervical cancer, ovarian cancer and metastatic breast cancer2.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · March 19, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY) today announced its consolidated financial results for the quarter and nine months ended December 31, 2023. The information mentioned in this release is based on consolidated financial statements under International Financial Reporting Standards (IFRS).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · January 31, 2024

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; hereafter referred to as “Dr. Reddy’s”), a global pharmaceutical company, has won back-to-back global recognitions for its commitment and progress on sustainability and Environment Social and Governance (ESG) agenda.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · December 14, 2023

Dr. Reddy’s Laboratories Ltd. (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY) today announced its consolidated financial results for the quarter ended Sep 30, 2023. The information mentioned in this release is based on consolidated financial statements under International Financial Reporting Standards (IFRS).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · October 27, 2023

Dr. Reddy’s Laboratories Ltd. (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY) today announced its consolidated financial results for the quarter ended June 30, 2023. The information mentioned in this release is based on consolidated financial statements under International Financial Reporting Standards (IFRS).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · July 26, 2023

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; hereafter referred to as “Dr. Reddy’s”), a global pharmaceutical company, announced that its Biologics License Application (BLA) for its proposed biosimilar rituximab candidate DRL_RI has been accepted for a substantive review by the U.S. Food and Drug Administration (USFDA). This closely follows acceptance of its rituximab biosimilar dossier for review by two other regulatory agencies – the European Medicines Agency (EMA) and the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · July 12, 2023

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; hereafter referred to as “Dr. Reddy’s”), a global pharmaceutical company, announced that its tocilizumab biosimilar candidate, DRL_TC, successfully met its primary and secondary endpoints in a Phase I study. This Phase I study used an intravenous (IV) formulation to evaluate the pharmacokinetic equivalence, safety and immunogenicity of Dr. Reddy’s tocilizumab biosimilar candidate in comparison to reference products.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · June 5, 2023
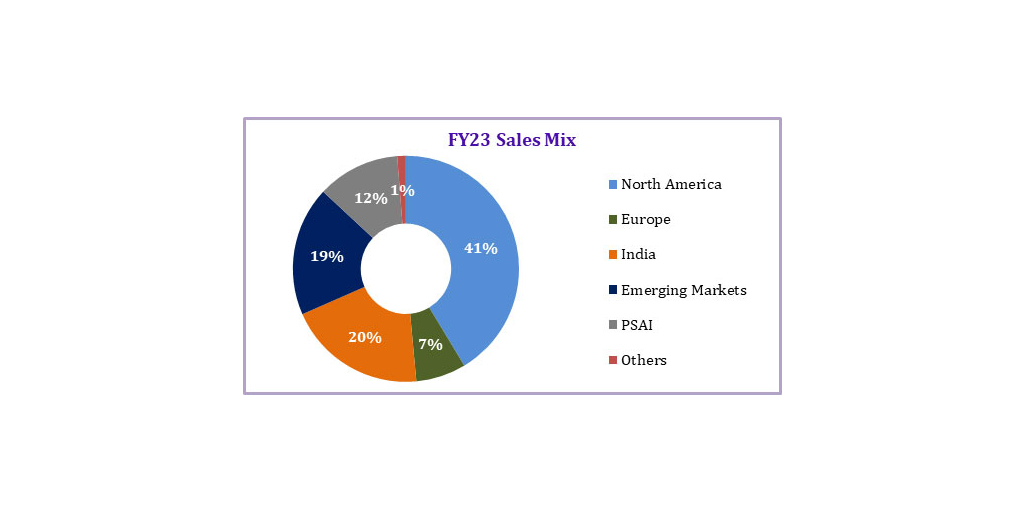
Dr. Reddy’s Laboratories Ltd. (BSE: 500124 | NSE: DRREDDY | NYSE: RDY | NSEIFSC: DRREDDY) today announced its consolidated financial results for the fourth quarter and full year ended March 31, 2023. The information mentioned in this release is on the basis of consolidated financial statements under International Financial Reporting Standards (IFRS).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · May 10, 2023

Dr. Reddy's Laboratories SA, wholly-owned subsidiary of Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced that it has entered into a definitive agreement to acquire the U.S. generic prescription product portfolio of Salisbury, Australia, based Mayne Pharma Group Limited (ASX: MYX).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · February 27, 2023

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, hereafter referred to as “Dr. Reddy’s”) announced major back-to-back recognitions in gender equality and sustainability.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · February 8, 2023

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Dr. Reddy’s Difluprednate Ophthalmic Emulsion 0.05%, a therapeutic generic equivalent to Durezol® (Difluprednate Ophthalmic Emulsion 0.05%) in the U.S. market, following the approval by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · January 27, 2023

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; hereafter referred to as “Dr. Reddy’s”), a global pharmaceutical company, announced that it has successfully completed the full set of clinical studies of its proposed rituximab biosimilar candidate, DRL_RI, for filing in highly regulated markets such as the United States, Europe and other regions.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · January 20, 2023

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; hereafter referred to as “Dr. Reddy’s”), a global pharmaceutical company, announced that its tocilizumab biosimilar candidate, DRL_TC, successfully met its primary and secondary endpoints in a Phase 1 study. This Phase 1 study used a subcutaneous formulation to evaluate the pharmacokinetic equivalence, safety and immunogenicity of Dr. Reddy’s tocilizumab biosimilar candidate in comparison to reference products.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · December 19, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; hereafter referred to as “Dr. Reddy’s”), a global pharmaceutical company, today announced the recognition of its largest manufacturing facility in Bachupally, Hyderabad, as part of the Global Lighthouse Network (GLN) by the World Economic Forum (WEF).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · October 11, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch, in the U.S. market, of Lenalidomide Capsules, a therapeutic equivalent generic version of REVLIMID® (lenalidomide) Capsules approved by U.S. Food and Drug Administration (USFDA). With this volume-limited launch, Dr. Reddy’s is eligible for first-to-market, 180 days of generic drug exclusivity for Lenalidomide Capsules in 2.5 mg and 20 mg strengths.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · September 7, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced that it has entered into a licensing agreement with Princeton, New Jersey based Slayback Pharma LLC (“Slayback”), to acquire rights in Slayback’s Brimonidine Tartrate Ophthalmic Solution 0.025%, the private label equivalent of Lumify® in U.S. Lumify® is an over-the-counter (OTC) eyedrop that can be used to relieve redness of the eye due to minor eye irritations. The agreement also provides Dr. Reddy’s exclusive rights to the product outside the U.S.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · July 29, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Bortezomib for Injection 3.5 mg, the generic equivalent of Velcade® (bortezomib) Injection, in the U.S. market approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · July 27, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) announces the launch of Dr. Reddy’s Fesoterodine Fumarate Extended-Release Tablets, a therapeutic generic equivalent to Toviaz® (fesoterodine fumarate) Extended-Release Tablets in the U.S. market following the approval by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · July 8, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced that it has acquired a portfolio of branded and generic injectable products from Deer Park, Illinois based-Eton Pharmaceuticals, Inc. (NASDAQETON).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · June 24, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Dr. Reddy’s Sorafenib Tablets, USP, 200 mg, a therapeutic generic equivalent of Nexavar® (sorafenib) Tablets in the U.S. market following the approval by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · June 14, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Pemetrexed for Injection, 100 mg and 500 mg Single-Dose Vials, the generic equivalent of ALIMTA® (pemetrexed for injection) in the U.S. Market approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · May 27, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) and Senores Pharmaceuticals, Inc. today announced the launch of Ketorolac Tromethamine Tablets USP, 10 mg, a therapeutic generic equivalent of the reference listed drug Toradol Tablets, 10 mg in the U.S. market approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · May 24, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Posaconazole Delayed-Release Tablets, 100 mg, the therapeutic generic equivalent to NOXAFIL® (posaconazole) Delayed-Release Tablets, 100 mg approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · April 20, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Methylprednisolone Sodium Succinate for Injection, USP, the generic equivalent of SOLU-MEDROL® (methylprednisolone sodium succinate for injection, USP) in the U.S. Market approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · April 5, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of its authorized generic version of Par Pharmaceutical’s VASOSTRICT® (vasopressin injection, USP) Vials in the U.S. Market approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · February 9, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, hereafter referred to as “Dr. Reddy’s”) announces two major back-to-back recognitions in sustainability and gender equality.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · February 2, 2022

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Venlafaxine ER Tablets which is therapeutically equivalent to Venlafaxine Extended-Release Tablets, 150 mg and 225 mg, of Osmotica Pharmaceutical US LLC approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · December 9, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Valsartan Tablets, USP, a therapeutic equivalent generic version of Diovan® (valsartan) Tablets approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · December 9, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Ephedrine Sulfate Injection USP, 50 mg/mL, a therapeutic equivalent generic version of Akovaz® (ephedrine sulfate injection) Injection, 50 mg/mL approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · October 29, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the final approval of its Abbreviated New Drug Application (ANDA) for Lenalidomide Capsules, in 2.5 mg and 20 mg strengths, and tentative approval for 5 mg, 10 mg, 15 mg, and 25 mg strengths, a therapeutic equivalent generic version of REVLIMID® (lenalidomide) Capsules, from the U.S. Food and Drug Administration (USFDA). With this approval, Dr. Reddy’s is eligible for 180 days of generic drug exclusivity for Lenalidomide Capsules, 2.5 mg and 20 mg.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · October 19, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Carmustine for Injection, USP, a therapeutic equivalent generic version of BiCNU® (carmustine for injection) approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · October 15, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”), announced that it has entered into a definitive agreement with Citius Pharmaceuticals, Inc. (“Citius”) pursuant to which it sold all of its rights to E7777 (an engineered IL-2-diphtheria toxin fusion protein) and certain related assets.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · September 4, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced that Reddy-Lenalidomide, a generic equivalent to Revlimid® (lenalidomide) capsules, is approved by Health Canada and has been launched in the Canadian market. Reddy-Lenalidomide is one of the first generic medications of its kind to launch in Canada.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · September 2, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Chlordiazepoxide Hydrochloride and Clidinium Bromide Capsules USP, 5 mg/2.5 mg, a therapeutic equivalent generic version of Librax® (chlordiazepoxide hydrochloride and clidinium bromide) approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · August 31, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the re-launch of over-the-counter (OTC) Naproxen Sodium Tablets USP, 220 mg, the store-brand equivalent of Aleve®, in the U.S. market, as approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · August 3, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Icosapent Ethyl Capsules, 1 gram approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · June 22, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Ertapenem for Injection, 1 g/vial, a therapeutic equivalent generic version of INVANZ (ertapenem for injection) for injection, 1 g/vial approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · May 12, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Albendazole Tablets, USP, a therapeutic equivalent generic version of Albenza Tablets, 200 mg, approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · April 29, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY) today announced that it has received the permission from the Drug Controller General of India (DCGI) to import the Sputnik vaccine into India for restricted use in emergency situations as per the provisions of the New Drug and Clinical Trials rules, 2019 under the Drugs and Cosmetics Act.
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · April 13, 2021

Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Sapropterin Dihydrochloride Powder for Oral Solution, 100 mg, a therapeutic equivalent generic version of Kuvan® (sapropterin dihydrochloride) Powder for Oral Solution, 100 mg, USP, approved by the U.S. Food and Drug Administration (USFDA).
By Dr. Reddy’s Laboratories Ltd. · Via Business Wire · April 7, 2021
