Articles from Can-Fite BioPharma Ltd.

Namodenoson’s Favorable Safety Profile and Broad Patent Portfolio Positions it as a Promising Candidate in the Growing Obesity Treatment Market
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · February 17, 2026

Namodenoson’s oral safety profile and metabolic activity, position it as a promising candidate in the rapidly growing obesity treatment market
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · February 9, 2026

Namodenoson Provided Clinical Stabilization as a Potential Bridge to Transplant in Advanced Liver Failure
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · February 5, 2026

Primary Safety Endpoint Demonstrated to Date; Top-Line Efficacy Data Expected in Q3 2026
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · January 20, 2026

RAMAT GAN, Israel, Dec. 26, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small-molecule drugs targeting oncological and inflammatory diseases, today announced that the Brazilian Patent Office (INPI) has granted Patent No. BR112015002697-4, entitled “Use of an A3 Adenosine Receptor Agonist for the Treatment of Sexual Dysfunction.”
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · December 26, 2025

RAMAT GAN, Israel, Dec. 23, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small-molecule drugs targeting oncological and inflammatory diseases, announced today that following the approval of its shareholders on November 10, 2025, its Board of Directors has approved a 1-for-3,000 reverse split of the Company’s ordinary shares. The reverse split will be recorded with the Tel-Aviv Stock Exchange on January 2, 2026 and on January 4, 2026, the Tel-Aviv Stock Exchange will be closed. The first trading date for the newly consolidated ordinary shares on the Tel-Aviv Stock Exchange will be January 5, 2026.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · December 23, 2025

RAMAT GAN, Israel, Dec. 16, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small-molecule drugs targeting oncological and inflammatory diseases, today announced an update on its clinical development activities and financial status.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · December 16, 2025

RAMAT GAN, Israel, Nov. 26, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs targeting oncological and inflammatory diseases, announced today that its CEO, Motti Farbstein will present at NobleCon21—Noble Capital Markets’ Twenty First Annual Emerging Growth Equity Conference—at Florida Atlantic University, Executive Education Complex, in Boca Raton, Floria—on Wednesday, December 3rd, 2025 at 12:30 PM ET. Mr. Farbstein will also conduct 1x1 meetings with investors.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · November 26, 2025

Namodenoson, a Phase III cancer drug has strong potential in veterinary oncology, a market projected to reach $3.1 billion by 2030
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · November 24, 2025

~35% of erectile dysfunction patients in a $3.2 billion market are non-responders to brands including Viagra and Cialis, and these drugs can be contraindicated for an estimated 16 million men living with diabetes
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · November 20, 2025

Pivotal Phase III liver cancer study enrolling in Europe and Israel
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · November 18, 2025

Ramat Gan, Israel, Sept. 15, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company developing a pipeline of proprietary small molecule drugs targeting oncological and inflammatory diseases, today announced a significant new clinical finding under its compassionate use program in decompensated liver cirrhosis. The patient, previously reported by Can-Fite to have experienced the disappearance of end-stage liver disease complications while receiving Namodenoson, has now demonstrated a complete resolution of esophageal varices, as confirmed by endoscopic evaluation.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · September 15, 2025

Ramat Gan, Israel, Aug. 28, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company developing a pipeline of proprietary small molecule drugs targeting oncological and inflammatory diseases, today announced financial results and clinical updates for H1, 2025.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · August 28, 2025

Primary endpoint is safety; Namodenoson continues to demonstrate a favorable safety profile
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · July 30, 2025

$5.0 million upfront with up to an additional $10.0 million of potential aggregate gross proceeds upon the exercise in full of short-term warrants
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · July 28, 2025

Ramat Gan, Israel, July 28, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a clinical-stage biotechnology company developing a pipeline of proprietary small molecule drugs for the treatment of cancer and inflammatory diseases, today announced that a leading group from University of Los Angeles (UCLA), demonstrate that Piclidenoson showed efficacy in an experimental model of vascular dementia.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · July 28, 2025

Ramat Gan, Israel, June 16, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a clinical-stage biotechnology company developing a pipeline of proprietary small molecule drugs for the treatment of cancer and inflammatory diseases, today announced that Dr. Sari Fishman, Vice President of Business Development, will present an update on the Company’s ongoing Phase IIa study in pancreatic cancer during partnering meetings at the 2025 BIO International Convention, taking place June 16–19 in Boston, MA (link).
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · June 16, 2025

Ramat Gan, Israel, May 05, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small-molecule drugs for oncological and inflammatory diseases, today announced that, it has raised $175 million in funding to date. These funds have enabled the advancement of its lead drug candidates, Namodenoson and Piclidenoson, into pivotal Phase III studies for liver cancer and psoriasis, respectively.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · May 5, 2025

Ramat Gan, Israel, April 17, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company developing a pipeline of proprietary small molecule drugs targeting oncological and inflammatory diseases, today announced that leading Medical Centers in the US are approaching the FDA, asking for compassionate use approval to treat patients with pancreatic carcinoma with the company oncological drug Namodenoson.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · April 17, 2025

RAMAT GAN, Israel, April 14, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small-molecule drugs for oncological and inflammatory diseases (“Can-Fite” or the “Company”), today announced that it has entered into definitive agreements for the purchase and sale of 2,500,000 of the Company’s American Depositary Shares (“ADSs”), at a purchase price of $1.20 per ADS, in a registered direct offering. The offering is expected to close on or about April 15, 2025, subject to satisfaction of customary closing conditions.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · April 14, 2025

Ramat Gan, Israel, April 14, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small-molecule drugs for oncological and inflammatory diseases, today announced the completion of a comprehensive analysis of its current partnerships and the market potential for its lead drug candidates, Piclidenoson and Namodenoson upon regulatory approvals.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · April 14, 2025

The psoriasis market is estimated at $30 Billion by 2030 and has shifted significantly to oral drugs
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · March 24, 2025

Can-Fite’s upfront and royalties on sales upon regulatory approval of Piclidenoson for veterinary use, is projected to be $325 million in the aggregate over the next decade assuming a 2029 launch
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · March 20, 2025

FDA & EMA approvals for rare genetic diseases are fast and require clinical studies with small number of patients
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · March 19, 2025

Ramat Gan, Israel, March 18, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company developing a pipeline of proprietary small molecule drugs targeting oncological and inflammatory diseases, today announced that it received a single FDA approval for the compassionate use treatment of a U.S. based pancreatic cancer patient with its anti-cancer drug Namodenoson.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · March 18, 2025

Unlike chemotherapy with its known toxicity towards normal body systems, Namodenoson provides protective effects
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · March 3, 2025

Liver cirrhosis treatment market is estimated to reach approximately $15 billion in the U.S. by 2030
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · February 18, 2025

RAMAT GAN, Israel, Feb. 10, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, invites investors to a webinar on Tuesday, February 25, 2025, at 4:15 p.m. ET.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · February 10, 2025

Ramat Gan, Israel, Feb. 05, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that it will present at the BIO CEO & Investor Conference, taking place February 10-11, 2025, in New York City.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · February 5, 2025

The Namodenoson oral drug is well positioned in the field of anti-obesity agents due to its activity and favorable safety profile
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · January 27, 2025

Ramat Gan, Israel, Dec. 30, 2024 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address Oncology and Inflammatory diseases, today announced that its work titled “The Liver Protective Effect of the anti-Cancer Drug Candidate Namodenoson is Mediated via Adiponectin” will be presented at the 2025 ASCO Gastrointestinal Cancers Symposium to take place at San Francisco & On Line, January 23-25.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · December 30, 2024

RAMAT GAN, Israel, Dec. 04, 2024 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, announced today that a patient currently treated with Namodenoson in a compassionate use program in Can-Fite’s Phase II Liver Cancer Study has an overall survival time of 8 years with a complete response.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · December 4, 2024

Orphan Drug Designation has been granted lately by US FDA
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · November 11, 2024

Namodenoson is an oral drug with a proven favorable safety profile
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · November 4, 2024

Data Reported by Can-Fite Veterinary Partner Vetbiolix who already exercised its option for a full license deal worth $325M
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · October 18, 2024

RAMAT GAN, Israel, Oct. 09, 2024 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced the Company’s oncology drug candidate, Namodenoson, has been granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) for the indication of pancreatic cancer, one of the most aggressive malignancies. The designation as an orphan drug will provide among others, potential for market exclusivity for seven years after approval and several and regulatory advantages (https://www.fda.gov/industry/medical-products-rare-diseases-and-conditions)
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · October 9, 2024

RAMAT GAN, Israel , Oct. 07, 2024 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, invites investors to a webinar on October 29, 2024, at 4:15 p.m. ET.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · October 7, 2024

The agreement has been signed upon successful conclusion of a clinical study in dogs with osteoarthritis
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · September 24, 2024

Study will aim to establish safety and clinical efficacy
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · September 16, 2024

RAMAT GAN, Israel, Aug. 08, 2024 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced the entry into a definitive agreement for the immediate exercise of certain outstanding warrants to purchase up to an aggregate of 2,857,143 American Depositary Shares (ADSs), having an exercise price of $1.75 per ADS, issued by Can-Fite in January 2023 and November 2023. The ADSs representing ordinary shares issuable upon exercise of the warrants are registered pursuant to effective registration statements on Form F-3 (File No. 333-276000) and Form F-1 (File No. 333-269485). The closing of the offering is expected to occur on or about August 12, 2024, subject to satisfaction of customary closing conditions.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · August 8, 2024

Broad Protection of Namodenoson is expected till at least 2044
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · July 29, 2024

RAMAT GAN, Israel, July 17, 2024 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CFBI), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, invites investors to a webinar on August 8, 2024, at 4:15 p.m. ET.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · July 17, 2024

PETACH TIKVA, Israel, July 11, 2024 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that it has submitted an application to the U.S. Food and Drug Administration (FDA) for Orphan Drug Designation for its drug candidate Namodenoson in the treatment of pancreatic carcinoma.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · July 11, 2024

Liver cirrhosis treatment global market is estimated to reach $29.2 billion by 2030
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · July 1, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CFBI), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that its veterinary partner Vetbiolix reported positive results in an osteoarthritis multicenter clinical study in dogs treated with Piclidenoson. Vetbiolix, Can-Fite’s veterinary commercialization partner which is covering all costs associated with veterinary clinical development, concluded successfully the study interim analysis.
By Can-Fite BioPharma Ltd. · Via Business Wire · June 28, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announces that company scientists came up with breakthrough findings showing that the anti-cancer and protective effects in the liver are conferred via the signalling protein adiponectin. This very important positive cytokine plays a pivotal role in regulating anti-inflammatory, anti-cancer, metabolic and insulin resistance. Namodenoson increases adiponectin production in pre-clinical studies and in humans.
By Can-Fite BioPharma Ltd. · Via Business Wire · June 24, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that it received an approval from the Institutional Review Board (IRB) of Rabin Medical Center, a leading medical institution in Israel where the study will be conducted. The approved protocol has been submitted now to the Ministry of Health (MOH).
By Can-Fite BioPharma Ltd. · Via Business Wire · June 10, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced an update on the status of its oncological lead drug candidate, Namodenoson in the treatment of advanced liver cancer. The Phase 3 pivotal study now has 31 recruiting medical centers in Europe, Israel and the US. Namodenoson has Orphan Drug status with both the U.S. Food and Drug Administration (FDA) and European Medicines Agency, as well as Fast Track Status with the FDA. A compassionate use program has also been ongoing in Israel and Romania.
By Can-Fite BioPharma Ltd. · Via Business Wire · June 5, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today reported that the International Journal of Molecular Sciences published a scientific review by independent scientists summarizing >50 publications from scientists all over the world stating that piclidenoson and namodenoson have a positive effect on heart diseases (Link to article).
By Can-Fite BioPharma Ltd. · Via Business Wire · May 29, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announces that its VP of Business Development, Dr. Sari Fishman, will hold 23 partnering meetings with Biotech & Pharma companies, who showed interest in partnering with Can-Fite. The meetings will take place during the Bio International Convention 2024 in San Diego, USA, on June 3-6. https://convention.bio.org/registration?gad_source=1&gclid=Cj0KCQjwu8uyBhC6ARIsAKwBGpR1uawA2qpi9I2O3htc7bwxwiKsbpmP1IefbWC8KrkYc5njVnODEpYaAsWWEALw_wcB
By Can-Fite BioPharma Ltd. · Via Business Wire · May 27, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, is pleased to invite investors to a webinar on June 6, 2024, at 4:15 p.m. ET.
By Can-Fite BioPharma Ltd. · Via Business Wire · May 23, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announces that it held a conference for 75 oncologists and coordinators who are conducting the pivotal Phase 3 advanced liver cancer study, to accelerate patient enrolment.
By Can-Fite BioPharma Ltd. · Via Business Wire · May 13, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announces that the U.S. Food and Drug Administration (FDA) has granted Investigational New Drug (IND) clearance for Namodenoson, for the treatment of patients with metabolic dysfunction-associated steatohepatitis (MASH), also known as non-alcoholic steatohepatitis (NASH), for the Company’s ongoing Phase IIb clinical study.
By Can-Fite BioPharma Ltd. · Via Business Wire · May 9, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced it received a Notice of Allowance from the European Patent Office for its patent application titled “An A3 Adenosine Receptor Ligands For Use in Treatment of a Sexual Dysfunction”. This invention addresses the use of the CF602 drug candidate as an oral or topical drug for patients such as diabetics, who suffer from erectile dysfunction, and cannot use the current drugs on the market.
By Can-Fite BioPharma Ltd. · Via Business Wire · May 6, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced it published an article in the Experimental and Therapeutic Medicine Journal, titled “Long-term complete response to namodenoson in liver cancer with Child-Pugh B cirrhosis: A case report” (Link). The patient participated in the Phase II Liver Cancer Study and has been treated with namodenoson for >7 years under compassionate use program.
By Can-Fite BioPharma Ltd. · Via Business Wire · April 25, 2024
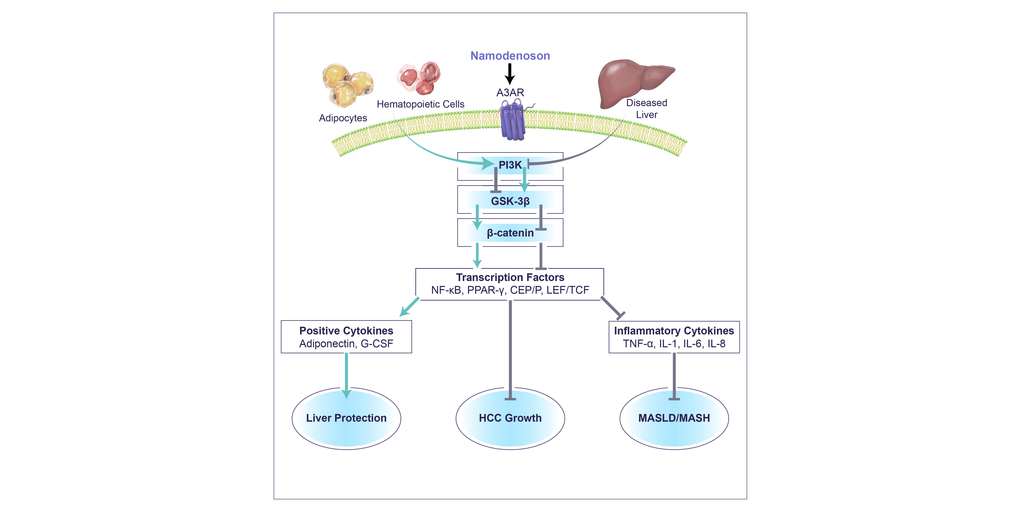
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that Biomedicines published an article titled “Namodenoson at the Crossroad of Metabolic Dysfunction-Associated Steatohepatitis (MASH) and Hepatocellular Carcinoma (HCC)”. Biomedicines is a highly reputable journal that publishes articles on clinical and basic science topics in medicine. https://www.mdpi.com/2227-9059/12/4/848.
By Can-Fite BioPharma Ltd. · Via Business Wire · April 15, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced submission of an investigational new drug (IND) application to the U.S. Food and Drug Administration (FDA) for the treatment of metabolic dysfunction-associated steatohepatitis (MASH), also known as non-alcoholic steatohepatitis (NASH), for the Company’s ongoing Phase IIb clinical study.
By Can-Fite BioPharma Ltd. · Via Business Wire · April 3, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced financial results and clinical updates for the twelve months ended December 31, 2023.
By Can-Fite BioPharma Ltd. · Via Business Wire · March 28, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncology and inflammatory diseases, today announced its VP of Business Development, Dr. Sari Fishman, will present data from the pancreatic and liver cancer programs during numerous partnering meetings at the BIO-Europe Spring 2024 in Barcelona, Spain, on Mar 18-27, 2024 (https://informaconnect.com/bioeurope-spring/).
By Can-Fite BioPharma Ltd. · Via Business Wire · March 11, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced it received a Notice of Allowance from the Canadian Intellectual Property Office for its patent application titled “An A3 Adenosine Receptor Ligand For Use In Treating Ectopic Fat Accumulation”. This invention addresses the use of Namodenoson for the reduction of liver fat in patients with NASH a clinical indication that is being developed by Can-Fite. In a successfully concluded Phase IIa study, Namodenoson, one of the Company’s two drugs in advanced clinical development, reduced liver fat content, showed anti-inflammatory effects manifested by a significant decrease in the liver enzymes ALT & AST, and decreased body weight in patients with NASH. A Company-sponsored study for Namodenoson for this indication is currently enrolling patients for a Phase IIb study which will include 140 patients, in whom liver pathology is the primary endpoint.
By Can-Fite BioPharma Ltd. · Via Business Wire · February 28, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that it has expanded its out licensing transaction with Ewopharma AG. to include the pancreatic cancer indication of Can-Fite.
By Can-Fite BioPharma Ltd. · Via Business Wire · January 30, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that the Journal of the European Academy of Dermatology and Venereology (EADV) published an article titled “Efficacy and safety of piclidenoson in plaque psoriasis: Results from a randomized phase 3 clinical trial (COMFORT-1)”. EADV is a top ranked peer reviewed journal (impact factor 9.2) that publishes articles on clinical and basic science topics in dermatology. Article Link.
By Can-Fite BioPharma Ltd. · Via Business Wire · January 29, 2024

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today reported new data on Namodenoson’s anti-obesity mechanism of action.
By Can-Fite BioPharma Ltd. · Via Business Wire · December 20, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that it received a positive response from the U.S. Food and Drug Administration (FDA) on the Pediatric Study Plan for the treatment of children suffering from psoriasis with Piclidenoson.
By Can-Fite BioPharma Ltd. · Via Business Wire · December 18, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that it completed the design of a Phase IIa study protocol for the treatment of patients with pancreatic cancer and plans to submit the protocol shortly to ethical committees for approval.
By Can-Fite BioPharma Ltd. · Via Business Wire · December 4, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced financial results for the nine months ended September 30, 2023.
By Can-Fite BioPharma Ltd. · Via Business Wire · November 30, 2023

PETACH TIKVA, Israel, Nov. 21, 2023 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced the entry into a definitive agreement for the immediate exercise of certain outstanding warrants to purchase up to an aggregate of 1,963,637 American Depositary Shares (ADSs), having exercise prices ranging from $6.00 to $5.50 per ADS, issued by Can-Fite in December 2021 and January 2023, at a reduced exercise price of $1.53 per share. The ADSs representing ordinary shares issuable upon exercise of the warrants are registered pursuant to effective registration statements on Form F-3 (File No. 333-262055) and Form F-1 (File No. 333-269485). The closing of the offering is expected to occur on or about November 24, 2023, subject to satisfaction of customary closing conditions.
By Can-Fite BioPharma Ltd. · Via GlobeNewswire · November 21, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today announced that a patient who participated in the Phase II Liver Cancer Study and was treated with namodenoson has a complete response and overall survival of 6.9 years (82.8 months).
By Can-Fite BioPharma Ltd. · Via Business Wire · November 21, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncological and inflammatory diseases, today confirmed that all of the Company’s clinical and business development activities are ongoing and proceeding by both its Israeli and US based staff.
By Can-Fite BioPharma Ltd. · Via Business Wire · November 1, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncology, inflammatory and liver diseases, today announced that Biomolecules, a peer-reviewed scientific journal focused on function and mechanism of bioactive molecules, published an article titled “Namodenoson Inhibits the Growth of Pancreatic Carcinoma via Deregulation of the Wnt/β-catenin, NF-κB, and RAS Signaling Pathways”, authored by Can-Fite’s CSO Dr. Pnina Fishman and others.
By Can-Fite BioPharma Ltd. · Via Business Wire · October 30, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncology and inflammatory diseases, today announced that it entered into an agreement with Collaborations Pharmaceuticals, Inc. (CPI) to develop anti-cancer drugs utilizing artificial intelligence (AI) and machine learning (ML) techniques. This project will aim to develop a next-generation A3 adenosine receptor drug agonists that significantly reduce the development time and cost of bringing such drugs to market.
By Can-Fite BioPharma Ltd. · Via Business Wire · October 26, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncology, inflammatory and liver diseases, today announced that the Company’s Director of Business Development Dr. Sari Fishman will conduct virtually one-on-one meetings with Japanese companies specializing in the development of Orphan Drugs, at the BioJapan Conference held from October 11-13, 2023, in Yokohama, Japan https://jcd-expo.jp/en/.
By Can-Fite BioPharma Ltd. · Via Business Wire · October 9, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncology, inflammatory and liver diseases, today announced that its study titled “Namodenoson Inhibits the Growth of Pancreatic Carcinoma via De-regulation of the Wnt/β-catenin Signaling Pathway” has been accepted for a poster presentation at the AACR Special Conference on Pancreatic Cancer, held from September 27-30, 2023, in Boston, Massachusetts.
By Can-Fite BioPharma Ltd. · Via Business Wire · September 27, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CFBI), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncology, inflammatory and liver diseases, today announced that the Company’s CEO Motti Farbstein will present at the H.C. Wainwright 25th Annual Conference. In addition to the presentation, he will conduct one-on-one meetings with investors at the conference.
By Can-Fite BioPharma Ltd. · Via Business Wire · September 7, 2023

Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address oncology, inflammatory and liver diseases, today announced financial results for the six months ended June 30, 2023.
By Can-Fite BioPharma Ltd. · Via Business Wire · August 31, 2023
