Articles from Biocytogen Pharmaceuticals (Beijing) Co., Ltd.

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen,” SSE: 688796; HKEX: 02315), a global biotechnology company advancing innovative drug discovery, today announced its successful listing on the Shanghai Stock Exchange STAR Market. This follows the Company’s listing on the Hong Kong Stock Exchange in September 2022 and marks a significant milestone in establishing Biocytogen as the first “H+A” dual-listed global drug innovator, reinforcing its mission to become the global headstream of new drugs.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · December 10, 2025

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (Biocytogen, HKEX: 02315), announced that its partner IDEAYA Biosciences, Inc. (Nasdaq: IDYA), a precision oncology company, has received the clearance of an investigational new drug (IND) application with the U.S. Food and Drug Administration (FDA) for the initiation of a Phase 1 clinical trial of IDE034, a potential first-in-class B7H3/PTK7 bispecific antibody-drug conjugate (ADC). IDEAYA expects to begin patient enrollment in Q1 2026, initially evaluating patients with solid tumors known to express B7H3 and PTK7, including lung, colorectal, head and neck, and ovarian/gynecological cancers.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · December 4, 2025

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (Biocytogen, HKEX: 02315), a global biotechnology company that drives the research and development of novel antibody-based drugs with innovative technologies, today announced that ADC therapeutics developer, Tubulis has exercised an exclusive license for the global development and commercialization of a fully human antibody developed by Biocytogen. The antibody will be applied in a novel ADC candidate proprietary to Tubulis. It is part of a previously signed research collaboration and option agreement to discover and advance antibody components for the development and commercialization of ADC products based on Biocytogen’s antibody discovery engine.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · September 15, 2025

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (Biocytogen, HKEX: 02315), a global biotechnology company that drives the research and development of novel antibody-based drugs with innovative technologies and Merck KGaA, Darmstadt, Germany, a leading science and technology company, have entered into an evaluation and option agreement to advance the development of antibody-conjugated lipid-based delivery solutions for nucleic acid payloads, such as antibody-conjugated lipid nanoparticle (LNP).
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · September 3, 2025

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (Biocytogen, HKEX: 02315), a global biotechnology company that drives the research and development of novel antibody-based drugs with innovative technologies, today announced that it has entered into a global licensing agreement with BeOne Medicines Ltd., a global oncology company, for multiple fully human antibodies.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · July 9, 2025

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (Biocytogen, HKEX: 02315) today announced that the key technology of its independently developed RenMab™ fully human antibody mouse platform has been granted an invention patent by the Japan Patent Office (JPO). This milestone marks a significant step in strengthening the global intellectual property portfolio of the RenMice® fully human antibody platform family. It underscores the continued advancement of Biocytogen’s comprehensive global patent strategy and highlights the innovation and international recognition of the company’s proprietary technologies.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · June 5, 2025

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) and Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd. (“NJCTTQ”) announced that NTB003 (formerly BCG009), a co-developed injectable drug candidate, has received the Investigational New Drug (IND) approval from the National Medical Products Administration (NMPA). The approved indication is Thyroid Eye Disease (TED).
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · June 4, 2025

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (Biocytogen, HKEX: 02315), a global biotech company focusing on the discovery of novel antibody/ADC therapeutics, announced that Adcendo ApS ("Adcendo"), a biotech company focused on the development of first-in-class antibody-drug conjugates (ADCs) for the treatment of cancers with high unmet medical need, has exercised its option under a research, option and license agreement to utilize Biocytogen’s fully human antibodies to further expand its ADC pipeline.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · December 16, 2024

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (Biocytogen, HKEX: 02315), a global biotech company focusing on the discovery of novel antibody/ADC therapeutics, announced that IDEAYA Biosciences, Inc. (Nasdaq: IDYA), a precision medicine oncology company committed to the discovery and development of targeted therapeutics, has exercised the option for an exclusive worldwide license for Biocytogen's B7H3/PTK7 BsADC program, IDE034, and has nominated it as a development candidate.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · November 11, 2024

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) announced United States Patent and Trademark Office (USPTO) patent grant for independently developed RenLite® fully human common light chain mouse platform.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · June 21, 2024

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315), a global biotechnology company focused on the discovery of novel antibody-based therapeutics, today announced a TCR-mimic antibody evaluation and potential licensing agreement with BioCopy AG (“BioCopy”), a research-based biotechnology company headquartered in Basel, Switzerland.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · May 6, 2024

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315), a global biotechnology company that drives the research and development of novel antibody-based drugs with innovative technologies, and ABL Bio Inc. (“ABL”, KOSDAQ: 298380), a Korean clinical-stage biotechnology company developing novel therapeutics in oncology and CNS diseases, today announced a collaboration to develop new bispecific antibody-drug conjugates (bsADCs).
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · March 25, 2024

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) today announces an antibody evaluation and option agreement with Gilead Sciences, Inc. The agreement provides Gilead access to Biocytogen’s extensive fully human antibody library generated against a wide range of therapeutic targets. Over a three-year nomination period, Gilead will nominate targets of interest and evaluate the corresponding antibodies, with the option to acquire selected antibodies for worldwide therapeutic development.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · February 20, 2024

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) today announced the launch of a new sub-brand, RenBiologics™, to represent the company’s antibody discovery business division. RenBiologics business will cover out-licensing/co-development of the company’s extensive library of fully human antibodies, as well as licensing of RenMice®, the company’s fully human antibody/TCR discovery platforms. The RenBiologics logo features an antibody with human-centric design elements, highlighting Biocytogen's expertise in discovering fully human antibodies; the encircled design underscores the company’s dedication to becoming a global resource of fully human antibodies to expedite the development of novel antibody-based therapeutics.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · January 23, 2024

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen,” HKEX: 02315), a global biotech company focusing on the discovery of novel antibody therapeutics, today announces that it has entered into an Exclusive Option and License Agreement with Radiance Biopharma Inc. (“Radiance”), a biotechnology company specializing in developing next generation Antibody Drug Conjugates.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · January 8, 2024

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) and CtM Biotech (Shanghai) Co., Ltd ("CtM Bio") today jointly announce that the development of a tri-specific T cell engager for an intracellular target achieved milestone progress. In just one year since the start of the collaboration, the companies have identified a WT1/HLA-A02-specific T cell engager (WT1xCD3x4-1BB tri-specific antibody) with outstanding preclinical anti-tumor activities for both hematological malignancies and solid tumors. The incorporation of the T co-stimulatory signal for WT1-targeting T cell engager further gives it the best-in-class properties and first-in-class potential. Its preclinical data has been accepted for presentation at the upcoming 2024 AACR annual meeting.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · December 27, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) today announced that it has entered into an antibody evaluation and option agreement with Neurocrine Biosciences, Inc. The agreement grants Neurocrine Biosciences access to Biocytogen’s fully human antibodies against multiple specified targets, with an option to license selected antibodies for therapeutic product development, manufacturing and commercialization for all uses worldwide. Additional targets may be included under this agreement subject to mutual agreement. The antibodies were generated by Biocytogen’s proprietary RenMice® platforms.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · December 19, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315), a global biotech company focusing on the discovery and development of novel antibody therapeutics, today announces an antibody evaluation, option and license agreement with Ona Therapeutics (“Ona”), a Spanish biotech company specialized in unravelling novel biology to design biopharmaceuticals attacking advanced cancer.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · December 18, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) announced today that the company will be presenting 16 posters and exhibiting at booth 223 at the Society for Immunotherapy of Cancer (SITC) annual conference, taking place from November 1-5, 2023 in San Diego, CA.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · October 24, 2023
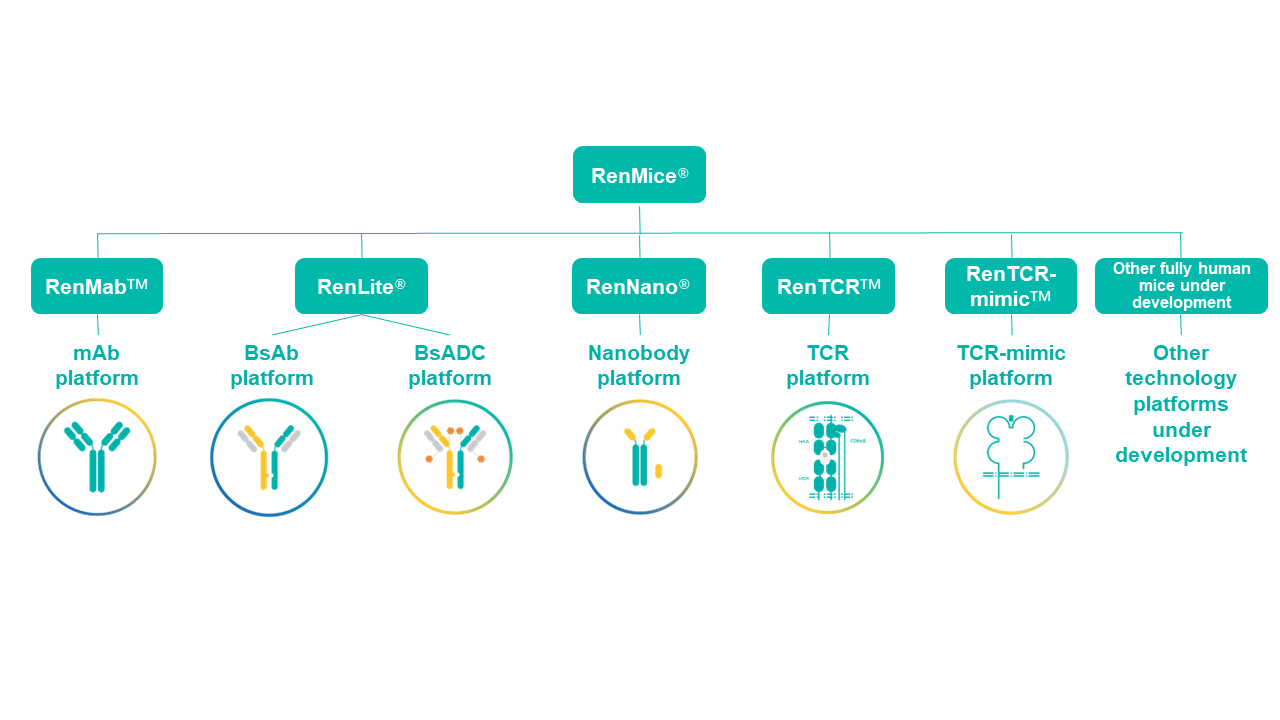
Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) officially announces the RenMice® series, which includes a collection of independently developed, fully human antibody mice and TCR mice with proprietary intellectual property. The term "Ren", defined as “human”, is derived from the Chinese pinyin for "人" (rén), which integrates Eastern cultural elements and represents Biocytogen's commitment to developing innovative technologies that advance the discovery, development, and delivery of novel therapeutics, ultimately benefiting human health. The RenMice® series encompasses five strains of fully human antibody/TCR mice: RenMab™, RenLite®, RenNano®, RenTCR™, and RenTCR-mimic™. These mice provide robust support for the discovery of fully human monoclonal antibodies, bispecific antibodies, bispecific antibody-drug conjugates (BsADCs), nanobodies, fully human T-cell receptors (TCRs), and TCR-mimic antibodies. Biocytogen is also generating more strains of fully human RenMice for MHC cluster genes, NK cell cluster genes, and other gene clusters for diverse drug development purposes, which will be integrated into the RenMice portfolio upon successful development.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · September 15, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315), a global biotech company focusing on the discovery of novel antibody therapeutics, today announces an antibody evaluation, option and license agreement with Myricx Bio (‘Myricx’), a UK biotech company focusing on the discovery and development of a completely novel class of selective cytotoxic payloads for antibody drug conjugates (ADCs).
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · September 7, 2023
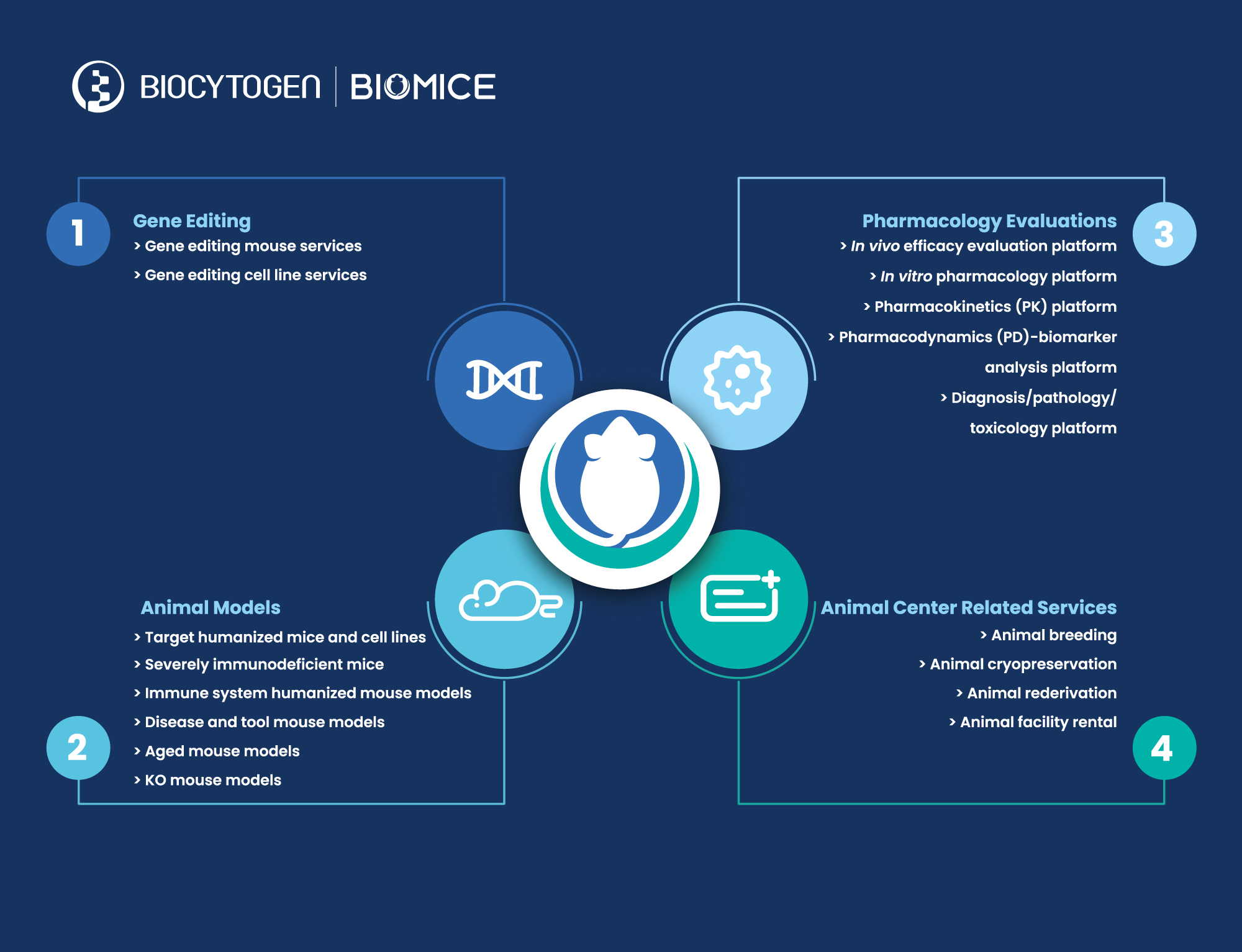
Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) today announces the official formation of two major business divisions to represent the company’s product, service, and asset portfolio. Over the last decade, Biocytogen has developed several technology platforms to streamline the entire drug development process, including generation of specialized (humanized) animal and cell model products, preclinical pharmacology evaluation, and most recently, fully human antibody discovery and clinical development. With the company’s expanding initiatives focused on antibody drug development, the company has officially established two business divisions, with one focusing on animal models and preclinical services, and the other focusing on the discovery and development of novel antibody-based drugs for out-licensing and partnerships.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · August 31, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) announced today that its Project Integrum has completed 2 core milestones, and its antibody business is now entering a new stage of rapid growth.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · August 17, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315), a global biotech company focusing on the discovery of novel antibody therapeutics, today announces an antibody license agreement with Pheon Therapeutics (Pheon), a leading Antibody-Drug Conjugate (ADC) specialist developing next generation ADCs for a wide range of hard-to-treat cancers. Under the terms of the agreement, Pheon will develop and commercialize an antibody developed using Biocytogen’s proprietary RenMice™ platforms. Biocytogen will receive an upfront payment and is eligible for development and commercial milestone payments, as well as single-digit royalties on net sales.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · July 5, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) has announced the opening of a new office in San Francisco, California, USA. The new space will aim to accelerate the globalization of Biocytogen’s businesses.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · May 31, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen") (HKEX: 02315), today announced the signing of a non-exclusive license agreement with Janssen Biotech, Inc., one of the Janssen Pharmaceutical Companies of Johnson & Johnson (“Janssen”). Under the agreement, Biocytogen grants Janssen and its affiliates a non-exclusive worldwide license to use Biocytogen’s proprietary RenLite® platform and underlying intellectual property to discover, research, develop, manufacture and commercialize fully human antibody therapeutics with a common light chain and other biological therapeutics for an unlimited number of drug targets and indications. The agreement was facilitated by Johnson & Johnson Innovation LLC.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · March 8, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) today announced that its wholly owned subsidiary, Eucure (Beijing) Biopharma Co., Ltd. (“Eucure Biopharma”), has reached an exclusive licensing agreement with Chipscreen NewWay Biosciences (“Chipscreen NewWay”), a holding subsidiary of Shenzhen Chipscreen Biosciences Co., Ltd. (“Chipscreen Biosciences”, SSE: 688321) for the clinical development and commercialization of bispecific antibody YH008 in Greater China (including Mainland China, Hong Kong, Macau and Taiwan). Eucure Biopharma retains YH008’s global rights to develop and commercialize YH008 outside Greater China. Under the agreement, Chipscreen NewWay will pay Eucure Biopharma an upfront payment of 40 million RMB, a potential development milestone payment of up to 360 million RMB, a potential sales milestone payment of up to 196 million RMB, as well as tiered royalties on net sales.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · February 27, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) today announced the launch of the “Nano 100 Project”, which aims to develop fully human nanobody drugs for over 100 targets. The Project combines Biocytogen’s proprietary fully human nanobody mouse, RenNano®, with its high-throughput in vitro and in vivo antibody screening platforms to develop fully human nanobody drugs on a large-scale.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · February 21, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) officially launched its fully human heavy chain antibody platform, RenNano®. RenNano® is the third member of the RenMice™ family, joining RenMab™ and RenLite®. Together, Biocytogen’s three RenMice™ platforms allow for the streamlined discovery and development of fully human monoclonal antibodies, bispecific/multispecific antibodies and single-domain antibodies (sdAbs, or nanobodies) (Figure 1).
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · January 5, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", SEHK : 02315) a clinical-stage biotechnology company with a focus on the antibody discovery and development based on its proprietary RenMiceTM Platforms, and Hansoh Pharmaceutical Group Company Limited (“Hansoh Pharma”, SEHK: 3692), a leading innovation-driven pharmaceutical company with a focus on the treatment of major diseases including oncology, infectious diseases, CNS disorders, metabolic diseases and autoimmune diseases, today announced an antibody collaboration, assignment and exclusive license agreement. Biocytogen will provide a license to Hansoh Pharma for their selected fully human antibody molecules against the designated target for the development, manufacturing and commercialization globally. Under the agreement, Biocytogen will receive an upfront payment and will be eligible to receive development and commercial milestones of up to tens of millions of Chinese yuan, as well as single-digit tiered royalties on net sales.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · January 3, 2023

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) today announced that the US FDA has approved the Investigational New Drug (IND) application for a phase I study of YH008, an internally developed first-in-class PD-1 x CD40 bispecific antibody (bsAb). The IND application was completed by Biocytogen’s wholly owned subsidiary Eucure Biopharma.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · December 19, 2022

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) today announced that it has entered into an evaluation and option agreement with ADC Therapeutics SA. Biocytogen will grant ADC Therapeutics a license to evaluate Biocytogen’s proprietary antibodies against three tumor targets, with an option to license selected antibodies at a later date for global ADC development and commercialization. Biocytogen reserves all global rights for these antibodies beyond ADC development. Biocytogen will receive an upfront payment. For each option exercised, Biocytogen will be entitled to an option-exercise fee, and development and commercial milestone payments, which potentially total tens of millions of US dollars, as well as single-digit royalties on net sales.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · November 28, 2022

Eucure (Beijing) Biopharma Co., Ltd. (“Eucure”), a wholly owned subsidiary of Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”), announced today that the company has entered into a worldwide licensing agreement with Syncromune, Inc. (“Syncromune”), a US-based clinical stage biopharmaceutical company, for the development and commercialization of intratumoral immunotherapies along with Syncromune’s Syncrovax™ technology.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · October 18, 2022

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen", HKEX: 02315) announced a strategic collaboration with Guangzhou FineImmune Biotechnology Co., LTD. (“FineImmune”) to co-develop cell-based therapeutic drugs targeting intracellular tumor-associated antigens. Biocytogen will use its proprietary TCR-mimic antibody platform to discover fully human antibody sequences that will be further developed using FineImmune’s unique cell therapy platform.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · September 15, 2022

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen") and TRACON Pharmaceuticals, Inc. (Nasdaq: TCON), today jointly announced that the U.S. Food and Drug Administration (FDA) has approved the Investigational New Drug (IND) application for the initiation of a Phase 1/2 clinical study of YH001 in combination with envafolimab and doxorubicin for the treatment of sarcoma patients, including patients who have not received prior therapy.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · August 29, 2022

Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen") entered into a global licensing agreement with Merck KGaA, Darmstadt, Germany, for the use of Biocytogen’s RenMiceTM platform. Under the terms of the agreement, Merck KGaA, Darmstadt, Germany will have full access to Biocytogen’s RenMiceTM platform to discover and develop fully human antibody therapeutics for an unlimited number of drug targets. Merck KGaA, Darmstadt, Germany will be responsible for all clinical development, manufacturing, and commercialization, and will provide Biocytogen with development and regulatory milestone payments. The official agreement was reached after an initial evaluation period.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · August 25, 2022

February 1st 2021, Biocytogen Pharmaceuticals (Beijing) Co., Ltd. ("Biocytogen") entered a strategic collaboration with LiberoThera Co., Ltd (“LiberoThera”) to co-develop fully human GPCR antibodies using Biocytogen’s advanced antibody discovery platform based on fully human antibody RenMabTM mice combined with LiberoThera’s outstanding membrane antigen preparation technology. In around a year since the collaboration was established, the two parties have screened out a number of fully human therapeutic antibody clones with excellent anti-tumor activity in vitro and in vivo against the first mutually selected GPCR target, CCR8, which has the potential to become a best-in-class product. These antibody clones exhibit high affinity binding to human CCR8 with species cross-reactivity and good manufacturability. Mechanistically, these clones can deplete Tregs from the tumor micro-environment through enhanced ADCC activity, and can also inhibit the activities of Tregs in the tumor micro-environment by inhibiting CCR8 signaling mediated by its ligand CCL1, thereby enhancing the anti-tumor immune response. In the future, the collaborations between two parties will also be extended to other GPCR targets.
By Biocytogen Pharmaceuticals (Beijing) Co., Ltd. · Via Business Wire · July 21, 2022
