Articles from BeiGene

BeiGene, Ltd. (NASDAQ: ONC; HKEX: 06160; SSE: 688235), a global oncology company that intends to change its name to BeOne Medicines Ltd., today announced that the Company will participate in fireside chats at two upcoming investor conferences:
By BeiGene · Via Business Wire · February 25, 2025
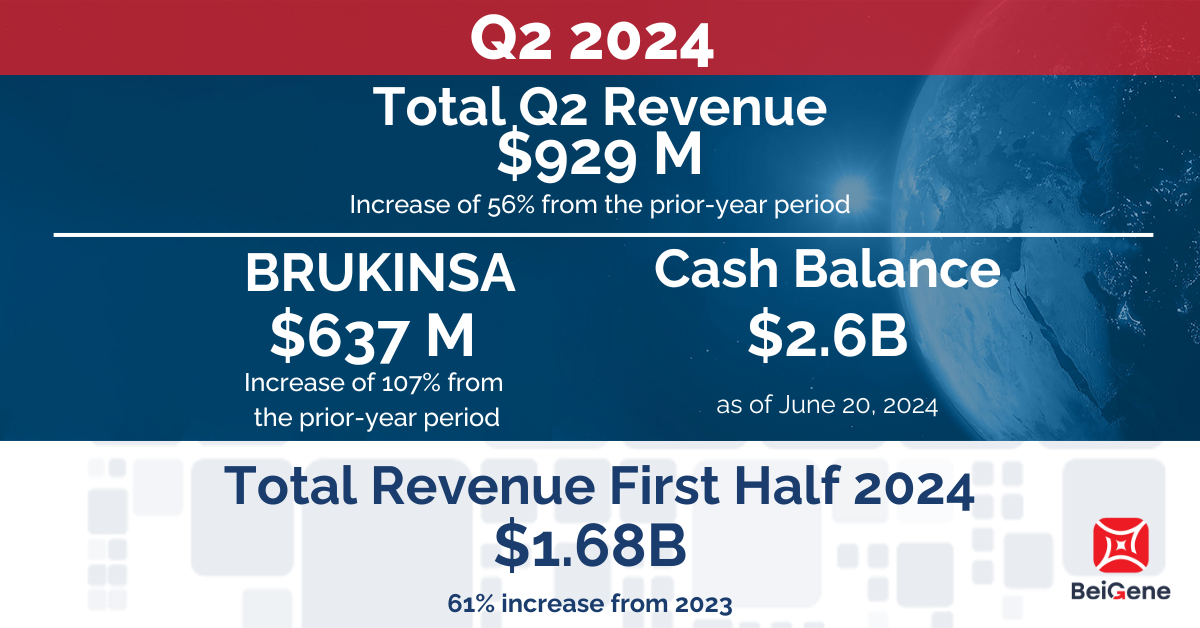
BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global oncology company, today announced results from the second quarter 2024 and corporate updates that strengthen the Company for future global growth.
By BeiGene · Via Business Wire · August 7, 2024

The Max Foundation (Max), a global nonprofit organization dedicated to accelerating health equity by delivering medication, technology, and supportive services to patients worldwide, BeiGene, a global oncology company, and the BeiGene Foundation, a nonprofit charitable foundation, today announced that the first doses of BRUKINSA® (zanubrutinib) have been administered for the treatment of adult patients with chronic lymphocytic leukemia (CLL) to patients in Armenia and Nepal, as part of a three-year collaboration to provide access to the medicine in 29 low- and middle-income countries (LMICs).
By BeiGene · Via Business Wire · March 13, 2024

BeiGene (Nasdaq: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a positive opinion recommending approval of BRUKINSA® (zanubrutinib), a Bruton’s tyrosine kinase inhibitor (BTKi), in combination with obinutuzumab for the treatment of adult patients with relapsed or refractory (R/R) follicular lymphoma (FL) who have received at least two prior lines of systemic therapy.
By BeiGene · Via Business Wire · October 13, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Company will participate in the Morgan Stanley 21st Annual Global Healthcare Conference on Monday, September 11th, 2023 with a fireside chat at 9:20 am ET.
By BeiGene · Via Business Wire · August 31, 2023

BeiGene (Nasdaq: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion recommending approval for tislelizumab as monotherapy for the treatment of adult patients with unresectable, locally advanced or metastatic esophageal squamous cell carcinoma (ESCC) after prior platinum-based chemotherapy.
By BeiGene · Via Business Wire · July 21, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced the U.S. Food and Drug Administration (FDA) has accepted for review the Company’s supplemental new drug application (sNDA) for BRUKINSA® (zanubrutinib) in combination with obinutuzumab for the treatment of adult patients with relapsed or refractory (R/R) follicular lymphoma (FL) after at least two prior lines of therapy. BRUKINSA was previously granted Fast Track and Orphan designation for this indication. The FDA has assigned a target action date in the first quarter of 2024, under the Prescription Drug User Fee Act.
By BeiGene · Via Business Wire · July 12, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, and DualityBio, a next-generation ADC company, today announced an agreement for BeiGene to acquire an exclusive option for a global clinical and commercial license to an investigational, preclinical ADC therapy for patients with select solid tumors.
By BeiGene · Via Business Wire · July 10, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Company will host an investor Research and Development Day in New York City and via webcast on July 18, 2023. John V. Oyler, BeiGene's Co-Founder, Chairman and CEO, along with the Company's leadership team, will provide an update on BeiGene’s deep and broad global innovation pipeline and platforms, and share insights on the Company's vision, differentiated capabilities, and value creation drivers.
By BeiGene · Via Business Wire · July 7, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, is aware that Pharmacyclics LLC has filed a complaint against BeiGene, Ltd. and BeiGene USA, Inc., alleging that BeiGene’s BRUKINSA® infringes a Pharmacyclics patent issued on June 13, 2023. BeiGene’s work is original, and we will vigorously defend against all allegations of patent infringement.
By BeiGene · Via Business Wire · June 15, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced the presentation of new data from its broad blood cancer portfolio of approved therapies and promising early-stage pipeline products at the 2023 European Hematology Association (EHA) Hybrid Congress. BeiGene has ten accepted abstracts at EHA, which is taking place from June 8-11 in Frankfurt, Germany.
By BeiGene · Via Business Wire · June 9, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced the presentation of new data showcasing the range of BeiGene’s research expertise and the productivity of one of the industry’s largest research and development teams at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago. These results include data for BeiGene’s cornerstone therapies, BRUKINSA® (zanubrutinib) and tislelizumab, as well as early results for BeiGene’s OX40 agonist and BCL-2 inhibitor.
By BeiGene · Via Business Wire · May 25, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Company will participate in fireside chats at two upcoming investor conferences:
By BeiGene · Via Business Wire · May 24, 2023

The Max Foundation (Max), a global nonprofit organization dedicated to accelerating health equity by delivering medication, technology, and supportive services to patients worldwide, BeiGene, a global biotechnology company, and the BeiGene Foundation, a nonprofit charitable foundation, today announced a collaboration to provide access to BRUKINSA (zanubrutinib) for the treatment of adult patients with chronic lymphocytic leukemia (CLL) in 29 low- and middle-income countries over the next three years. This collaboration advances each organization’s focus on patient access by combining Max’s expertise and infrastructure with a donated product from BeiGene, and a grant from the BeiGene Foundation.
By BeiGene · Via Business Wire · May 17, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced the China National Medical Products Administration (NMPA) approved four applications for BRUKINSA (zanubrutinib), the company’s Bruton’s tyrosine kinase inhibitor (BTKi), including two Supplemental New Drug Applications for treatment-naïve adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) and Waldenström's macroglobulinemia (WM), and two Supplemental Applications for conversions from conditional approval to regular approval.
By BeiGene · Via Business Wire · May 6, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, will present data from its broad solid tumor and hematology portfolio at the upcoming American Society of Cancer Oncology (ASCO) Annual Meeting in Chicago, June 2-6, 2023.
By BeiGene · Via Business Wire · April 26, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, announced several new Environmental, Social, and Governance (ESG) goals today, including targets related to carbon emissions and workforce diversity. These goals are outlined in the Company’s newly released “Change Is the Cure: 2022 Environmental, Social and Governance Report.” The report highlights BeiGene’s ESG strategy, the progress the Company has made against past goals, and how it intends to achieve its bold new targets.
By BeiGene · Via Business Wire · April 25, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced the global RATIONALE 305 trial met its primary endpoint of overall survival, with tislelizumab in combination with chemotherapy demonstrating superior overall survival (OS) compared with chemotherapy in patients with advanced unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma, regardless of PD-L1 status. No new safety signals were identified for tislelizumab.
By BeiGene · Via Business Wire · April 20, 2023

MapKure, LLC, BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), and SpringWorks Therapeutics, Inc. (NASDAQ: SWTX), today announced that they will present updated clinical data from the Phase 1a/1b study of BGB-3245, an investigational, selective RAF dimer inhibitor, in adult patients with advanced or refractory solid tumors harboring MAPK pathway aberrations. The data are being presented today in an oral presentation at the American Association for Cancer Research (AACR) Annual Meeting 2023, taking place in Orlando, Florida.
By BeiGene · Via Business Wire · April 17, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced the first major step in the company’s expansion in Latin America (LATAM) with the formal opening of its office in São Paulo, Brazil.
By BeiGene · Via Business Wire · April 13, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced the appointment of Julius Pryor III as its first Global Head of Diversity and Health Equity, effective immediately.
By BeiGene · Via Business Wire · March 30, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, is entering a new phase as it continues to build its U.S. presence. The company announced today that the last piece of structural steel will be laid at its Hopewell, NJ campus today. This new facility will provide state-of-the-art commercial-stage U.S. biologic pharmaceutical manufacturing, late-stage research and clinical development capabilities that complement the company’s existing capabilities around the world.
By BeiGene · Via Business Wire · March 21, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the China National Medical Products Administration (NMPA) granted approval for the company’s PD-1 inhibitor, tislelizumab, in combination with fluoropyrimidine and platinum chemotherapy, for the first-line treatment of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma with high PD-L1 expression.
By BeiGene · Via Business Wire · February 24, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Company will participate in Cowen’s 43rd Annual Healthcare Conference on Monday, March 6th, 2023 with a fireside chat at 11:10 am ET.
By BeiGene · Via Business Wire · February 24, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, has released a report highlighting the challenges cancer patients and caregivers face in managing mental and emotional well-being. The data summarized in the report is based on a BeiGene-sponsored study by Cancer Support Community (CSC), a global nonprofit organization that provides free emotional support, navigation, and resources to cancer patients and their loved ones. The report and the newly launched CancerandMentalHealth.com website are part of the company’s comprehensive Talk About It: Cancer and Mental Health program, designed to elevate the important intersection of mental health and cancer care.
By BeiGene · Via Business Wire · February 3, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Company will participate in two upcoming investor conferences:
By BeiGene · Via Business Wire · February 1, 2023
BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the U.S. Food and Drug Administration (FDA) has approved its Bruton’s tyrosine kinase inhibitor (BTKi) BRUKINSA (zanubrutinib) for the treatment of adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
By BeiGene · Via Business Wire · January 19, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorizations for BRUKINSA (zanubrutinib) in Great Britain for both the treatment of adult patients with chronic lymphocytic leukemia (CLL) and the treatment of adult patients with marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based therapy.
By BeiGene · Via Business Wire · January 19, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the National Reimbursement Drug List (NRDL) released by China’s National Healthcare Security Administration (NHSA) has been updated to include four new indications for its PD-1 inhibitor tislelizumab. KYPROLIS® (carfilzomib), a proteosome inhibitor licensed-in from Amgen, is included for the first time and XGEVA® (denosumab), a RANKL inhibitor and another Amgen asset, successfully renewed this year. The updated NRDL will officially take effect on March 1, 2023.
By BeiGene · Via Business Wire · January 18, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, will share clinical data and patient-reported outcomes for its PD-1 inhibitor, tislelizumab, at the 2023 ASCO Gastrointestinal Cancers Symposium, notably, an oral presentation for interim results of the global pivotal phase 3 trial RATIONALE 305 of tislelizumab in combination with chemotherapy in first-line gastric or gastroesophageal junction (G/GEJ) cancer. At the interim analysis, RATIONALE 305 trial met one of the primary endpoints of overall survival (OS) in patients with G/GEJ whose tumors express PD-L1.
By BeiGene · Via Business Wire · January 17, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company today announced that the Company will participate in the J.P. Morgan 41st Annual Healthcare Conference on Monday, January 9, 2023 at 1:30 p.m. PT.
By BeiGene · Via Business Wire · January 4, 2023

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company announced that the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) has accepted a supplemental biologics license application (sBLA) for tislelizumab in patients with first-line unresectable or metastatic hepatocellular carcinoma (HCC).
By BeiGene · Via Business Wire · December 30, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235) a global biotechnology company, today presented the final progression-free survival (PFS) analysis of the ALPINE trial demonstrating superior efficacy and a favorable cardiac safety profile for patients receiving BRUKINSA® as compared to IMBRUVICA® in a global phase 3 trial in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL). These data will be presented (Abstract #LBA-6) during the late-breaking session at the 64th American Society of Hematology (ASH) Annual Meeting in New Orleans and simultaneously published in The New England Journal of Medicine. The paper’s lead author Jennifer Brown, M.D., Ph.D., Director, CLL Center at Dana-Farber Cancer Institute will present these data.
By BeiGene · Via Business Wire · December 13, 2022

BeiGene, a global biotechnology company, is convening diverse stakeholders to amplify the mental health needs of the cancer community during an event hosted for patient advocates and scientists attending the American Society of Hematology (ASH) Annual Meeting and Exposition in New Orleans. In collaboration with Cancer Support Community (CSC), the company will preview new national survey findings1, including that 60 percent of individuals impacted by cancer who experienced emotional distress were not referred to a mental health professional by their cancer care team, and one in five who specifically wanted mental health support did not receive it.
By BeiGene · Via Business Wire · December 9, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235) a global biotechnology company, today announced that the results of the final progression free survival (PFS) analysis of the ALPINE trial will be presented at a late-breaking oral presentation session at the 64th American Society of Hematology (ASH) Annual Meeting in New Orleans. ALPINE is a global Phase 3 trial comparing BRUKINSA (zanubrutinib) with IMBRUVICA® (ibrutinib) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL). The results will be presented at 10:15 am CST during the late-breaking abstract session on Tuesday, December 13, 2022 in the Ernest N. Morial Convention Center, Hall E.
By BeiGene · Via Business Wire · November 22, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the European Commission (EC) has approved BRUKINSA® (zanubrutinib) for the treatment of adult patients with treatment-naïve (TN) or relapsed/refractory (R/R) CLL.
By BeiGene · Via Business Wire · November 17, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced the launch of a new program, Talk About It: Cancer and Mental Health, designed to elevate the important intersection of mental health and cancer care to help improve health outcomes for cancer patients.
By BeiGene · Via Business Wire · November 15, 2022

BeiGene, (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology, today announced BRUKINSA (zanubrutinib) has been approved in Brazil for the treatment of adult patients with Waldenström’s macroglobulinemia (WM) and adult patients with relapsed/refractory (R/R) marginal zone lymphoma (MZL) who have received at least one anti-CD20-based regimen. BRUKINSA was previously approved as a treatment in Brazil for adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. BeiGene is focused on developing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide.
By BeiGene · Via Business Wire · November 10, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the Company will participate in two upcoming investor conferences:
By BeiGene · Via Business Wire · November 4, 2022

BeiGene, (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced clinical and real-world data accepted at the 64th American Society of Hematology (ASH) Annual Meeting, scheduled for December 10-14, 2022, in New Orleans. BeiGene is focused on developing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide.
By BeiGene · Via Business Wire · November 3, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company, today announced that the European Commission (EC) has granted marketing authorization of BRUKINSA® (zanubrutinib) for the treatment of adult patients with relapsed/refractory (R/R) marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based therapy. The approval is applicable to all 27 member states of the European Union (EU), plus Iceland and Norway. BeiGene is focused on developing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide.
By BeiGene · Via Business Wire · November 2, 2022

BeiGene, (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company focused on developing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide, today announced significant progress in efforts to unlock global opportunities for BRUKINSA® (zanubrutinib) with recent regulatory approvals in six Latin American countries:
By BeiGene · Via Business Wire · October 26, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company focused on developing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide, today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion recommending approval of BRUKINSA® (zanubrutinib) for the treatment of adult patients with chronic lymphocytic leukemia (CLL).
By BeiGene · Via Business Wire · October 14, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company focused on developing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide, today announced that BRUKINSA® (zanubrutinib) achieved superior Progression-Free Survival (PFS) versus IMBRUVICA® (ibrutinib) in a final analysis of the Phase 3 ALPINE trial, as assessed by an independent review committee (IRC) and investigator. BRUKINSA was generally well tolerated; safety findings at the final PFS analysis were consistent with prior reports.
By BeiGene · Via Business Wire · October 12, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company that is developing and commercializing oncology medicines, today announced that England’s health technology assessment institute, the National Institute for Health and Care Excellence (NICE), has issued a final appraisal document (FAD) recommending BRUKINSA (zanubrutinib) for the treatment of Waldenström’s Macroglobulinemia (WM) in adults who have had at least one treatment, only if bendamustine plus rituximab is also suitable.
By BeiGene · Via Business Wire · September 19, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company focused on developing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide, today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion recommending approval of BRUKINSA ® (zanubrutinib) for the treatment of adult patients with marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based therapy.
By BeiGene · Via Business Wire · September 19, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, shared updates from its solid tumor development program for cornerstone PD-1 antibody tislelizumab at the European Society for Medical Oncology (ESMO) Congress 2022 in Paris.
By BeiGene · Via Business Wire · September 10, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company focused on developing and commercializing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide, today announced that the Company will participate in the Morgan Stanley 20th Annual Global Healthcare Conference on Wednesday, September 14th, 2022 with a fireside chat at 9:10 a.m. ET.
By BeiGene · Via Business Wire · September 7, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company focused on developing and commercializing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide, today announced that the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) has accepted a supplemental biologics license application (sBLA) for tislelizumab in combination with chemotherapy as first-line treatment in patients with unresectable locally advanced, recurrent or metastatic esophageal squamous cell carcinoma (ESCC).
By BeiGene · Via Business Wire · August 23, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company that is developing and commercializing innovative and affordable oncology medicines to improve treatment outcomes and access for far more patients worldwide, today announced a strategic agreement with Ontada®, a McKesson business with leading provider technology and actionable real-world research, education, and evidence in oncology, to improve U.S. community oncology care through the development of real-world evidence (RWE) data, tools, and insights to help increase access to affordable, cutting-edge therapies.
By BeiGene · Via Business Wire · August 15, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company focused on developing innovative and affordable oncology medicines to improve treatment outcomes and access for patients worldwide, today announced that the global Phase 3 RATIONALE 301 trial with tislelizumab met its primary endpoint of non-inferior Overall Survival (OS) versus sorafenib as a first-line treatment in adult patients with unresectable hepatocellular carcinoma (HCC). The safety profile for tislelizumab was consistent with previous studies and no new safety signals were reported. More than 600 patients in the U.S., Europe, and Asia participated in the study.
By BeiGene · Via Business Wire · August 9, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global oncology biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that the U.S. Food and Drug Administration (FDA) has deferred action on the Biologics License Application (BLA) for tislelizumab as a second-line (2L) treatment for patients with unresectable or metastatic esophageal squamous cell carcinoma (ESCC). The FDA has been unable to conduct required inspections in China due to COVID-19 related travel restrictions. As a result, the FDA is deferring action on the application until the inspections are complete.
By BeiGene · Via Business Wire · July 14, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global oncology biotechnology company focused on developing and commercializing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced the appointment of Chan Lee as General Counsel, effective July 18, 2022.
By BeiGene · Via Business Wire · July 13, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced it entered into a worldwide strategic collaboration with InnoRNA, a biotechnology company with expertise in LNP-based delivery technology and mRNA drug discovery, to leverage its innovative technology platform for developing mRNA-based therapeutics.
By BeiGene · Via Business Wire · July 6, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced new data from RATIONALE 306, a global Phase 3 trial evaluating tislelizumab plus chemotherapy in adult patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC) without prior systemic treatment for advanced disease. Study results presented today as a late-breaking oral presentation at the 2022 European Society for Medical Oncology (ESMO) World Congress on Gastrointestinal Cancer (Abstract #LBA-1) showed a statistically significant and clinically meaningful improvement in overall survival (OS) for patients receiving tislelizumab in combination with chemotherapy with a median OS of 17.2 months [95% CI: 15.8,20.1] versus 10.6 months [95% CI: 9.3,12.1] for those receiving chemotherapy plus placebo. The combination of tislelizumab with chemotherapy reduced the risk of death by 34% (HR=0.66 [95% CI: 0.54,0.80, p<0.0001]) compared to chemotherapy plus placebo.
By BeiGene · Via Business Wire · June 30, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) has accepted a supplemental biologics license application (sBLA) for the company’s anti-PD-1 inhibitor, tislelizumab, in combination with chemotherapy as a first-line treatment for patients with advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1.
By BeiGene · Via Business Wire · June 21, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide today announced that its BTK inhibitor BRUKINSA™ (zanubrutinib) has been approved by the Ministry of Health in Kuwait, the National Health Regulatory Authority in Bahrain and the Ministry of Public Health in Qatar for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. BeiGene is working with NewBridge Pharmaceuticals, a specialty company in the Middle East and North Africa (MENA) regions established to bridge the access gap by partnering with global pharma and biotech companies, to bring BRUKINSA to patients in Kuwait, Bahrain, Qatar, Saudi Arabia, United Arab Emirates, and other markets in the MENA region following regulatory approvals.
By BeiGene · Via Business Wire · June 13, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that the U.S. Food and Drug Administration (FDA) has extended the Prescription Drug User Fee Act (PDUFA) goal date by three months to January 20, 2023 for the supplementary new drug application (sNDA) for BRUKINSA as a treatment for adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
By BeiGene · Via Business Wire · June 13, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, presents data from its hematology portfolio at the European Hematology Association (EHA) 2022 Hybrid Congress being held June 9-12, 2022, in Vienna, Austria.
By BeiGene · Via Business Wire · June 10, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that the China National Medical Products Administration (NMPA) has approved BeiGene’s anti-PD-1 antibody, tislelizumab, in combination with chemotherapy as a first-line treatment for patients with recurrent or metastatic nasopharyngeal cancer (NPC).
By BeiGene · Via Business Wire · June 10, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that Julia Wang, Chief Financial Officer, and Angus Grant, Ph.D., Chief Business Executive, will participate in a fireside chat at the Goldman Sachs 43rd Annual Global Healthcare Conference on Tuesday, June 14, 2022, at 8:40 a.m. PT.
By BeiGene · Via Business Wire · June 8, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, will present data from its broad solid tumor and hematology portfolios in eight presentations at the upcoming American Society of Cancer Oncology (ASCO) Annual Meeting being held in Chicago from June 3-7, 2022. Highlights include new clinical data for its BTK-inhibitor zanubrutinib (BRUKINSA®):
By BeiGene · Via Business Wire · May 26, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced 20 presentations from the Company’s global clinical development programs in hematologic malignancies at the European Hematology Association (EHA) 2022 Hybrid Congress being held June 9 – 12, 2022 in Vienna, Austria.
By BeiGene · Via Business Wire · May 12, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that the China National Medical Products Administration (NMPA) has granted conditional approval of BLINCYTO® (blinatumomab) for injection for the treatment of pediatric patients with relapsed or refractory (R/R) CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). The NMPA granted conditional approval for adult patients in this indication in December 2020.
By BeiGene · Via Business Wire · May 4, 2022

BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced the groundbreaking of its flagship U.S. manufacturing and clinical R&D center at the Princeton West Innovation Campus in Hopewell, N.J.
By BeiGene · Via Business Wire · April 29, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that the BTK inhibitor BRUKINSA (zanubrutinib) has been approved in Uruguay for the treatment of adult patients with previously treated mantle cell lymphoma (MCL), relapsed or refractory marginal zone lymphoma (MZL), and Waldenström’s macroglobulinemia (WM). BeiGene and Adium entered into an exclusive distribution agreement for Adium to commercialize BRUKINSA in Latin America.
By BeiGene · Via Business Wire · April 28, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced presentations from the Company’s global clinical development programs in hematologic malignancies and solid tumors at the 2022 Annual Meeting of the American Society of Cancer Oncology (ASCO) being held on June 3-7, 2022.
By BeiGene · Via Business Wire · April 27, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced the Independent Data Monitoring Committee (IDMC) determined at a pre-planned interim analysis that RATIONALE 306, a global Phase 3 trial of tislelizumab in combination with chemotherapy, had met the study’s primary endpoint of overall survival (OS) in patients with previously untreated advanced or metastatic esophageal squamous cell carcinoma (ESCC). The safety and tolerability profile for tislelizumab in combination with chemotherapy at this interim analysis was consistent with previous trials and no new safety signals were identified.
By BeiGene · Via Business Wire · April 27, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines, and Crossroads4Hope, A Network of Cancer Support, a 501(c)(3) organization committed to transforming the cancer experience for patients and their families across New Jersey, today announced a grant from BeiGene that will enable Crossroads4Hope to expand the reach of its psychosocial support programs to more vulnerable patients and communities.
By BeiGene · Via Business Wire · April 21, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced the presentation of updated data analyses from the Phase 3 RATIONALE-309 trial of tislelizumab, a humanized anti-PD-1 monoclonal antibody, in combination with chemotherapy versus chemotherapy plus placebo as a first-line treatment for patients with recurrent or metastatic nasopharyngeal cancer (RM-NPC), at the virtual American Society of Clinical Oncology (ASCO) Plenary Series on April 19, 2022. Updated efficacy analyses showed that, at a median follow-up of 15.5 months, tislelizumab in combination with chemotherapy continued to demonstrate a clinically significant progression-free survival (PFS) benefit over chemotherapy alone for patients with RM-NPC. The safety profile of the tislelizumab and chemotherapy combination was generally manageable and consistent with known risks of each treatment agent.
By BeiGene · Via Business Wire · April 19, 2022

BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that the China National Medical Products Administration (NMPA) has granted approval to BeiGene’s anti-PD-1 antibody, tislelizumab, as a treatment for patients with locally advanced or metastatic esophageal squamous cell carcinoma (ESCC) who have disease progression or are intolerant to first-line standard chemotherapy.
By BeiGene · Via Business Wire · April 15, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that clinical results, as well as biomarker data, from its immuno-oncology program in solid tumors will be presented at the American Academy for Cancer Research (AACR) Annual Meeting 2022. The AACR meeting will take place April 8-13, 2022, as a hybrid event in New Orleans and in a virtual format.
By BeiGene · Via Business Wire · April 8, 2022

BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that marketing authorization applications (MAA) for tislelizumab, submitted by Novartis, the license holder in Europe, have been validated for regulatory review by the European Medicines Agency (EMA) for patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC) after prior systemic chemotherapy and for patients with non-small cell lung cancers (NSCLC) including:
By BeiGene · Via Business Wire · April 6, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160, SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, and Medison Pharma Ltd. a global pharma company focused on providing access to highly innovative therapies to patients in international markets ("Medison"), today announced that the State of Israel Ministry of Health has approved BRUKINSA® (zanubrutinib) for the treatment of adult patients with Waldenström’s Macroglobulinemia (WM). BRUKINSA is reimbursed nationally for patients with second- or third-line WM. This is the second approval for BRUKINSA in Israel, following its initial marketing and reimbursement approval in 2021 for patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
By BeiGene · Via Business Wire · March 15, 2022

BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced that the European Medicines Agency (EMA) has accepted for review two new indication applications for its BTK inhibitor BRUKINSA® (zanubrutinib) for the treatment of patients with chronic lymphocytic leukemia (CLL) and for the treatment of patients with marginal zone lymphoma (MZL).
By BeiGene · Via Business Wire · February 22, 2022
