Articles from Aldeyra Therapeutics, Inc.

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate virtually in a fireside chat at the Oppenheimer 36th Annual Healthcare Life Sciences Conference.
By Aldeyra Therapeutics, Inc. · Via Business Wire · February 18, 2026

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced that the U.S. Food and Drug Administration (FDA) has extended the Prescription Drug User Fee Act (PDUFA) target action date for the reproxalap New Drug Application (NDA) for the treatment of dry eye disease. The extended PDUFA target action date is March 16, 2026.
By Aldeyra Therapeutics, Inc. · Via Business Wire · December 15, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced at a research and development webcast the expansion of the RASP platform to include programs in central nervous system diseases associated with inflammation, and provided updated manufacturing information on reproxalap.
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 13, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the 2025 Jefferies Global Healthcare Conference in London, England.
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 11, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies for the treatment of immune-mediated diseases, today announced that the company will host a Research & Development Update webcast on Thursday, November 13, 2025 at 8:00 a.m. ET.
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 6, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced achievement of statistically significant improvement in liver function in patients treated with ADX-629, an investigational new drug candidate, and focused the RASP modulator product candidate pipeline on next-generation molecules ADX-248 and ADX-246.
By Aldeyra Therapeutics, Inc. · Via Business Wire · October 28, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the H.C. Wainwright 27th Annual Global Investment Conference on September 8, 2025.
By Aldeyra Therapeutics, Inc. · Via Business Wire · September 3, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that the European Medicines Agency (EMA) has granted Orphan Designation for ADX-2191 (methotrexate intravitreal injection, USP) for the treatment of primary large B-Cell lymphomas of immune-privileged sites, including primary vitreoretinal lymphoma. There is currently no approved treatment for patients with primary vitreoretinal lymphoma, a rare, aggressive, high-grade cancer that affects approximately 100 to 200 people per year in the European Union.
By Aldeyra Therapeutics, Inc. · Via Business Wire · August 28, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that the U.S. Food and Drug Administration (FDA) has granted fast track designation for ADX-2191 (methotrexate intravitreal injection, USP) for the treatment of retinitis pigmentosa. There is currently no approved treatment for patients with most forms of retinitis pigmentosa, a clinical group of rare genetic eye diseases characterized by retinal cell death and loss of vision. Retinitis pigmentosa affects more than one million people worldwide.
By Aldeyra Therapeutics, Inc. · Via Business Wire · August 19, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the H.C. Wainwright 5th Annual Ophthalmology Virtual Conference on August 13, 2025.
By Aldeyra Therapeutics, Inc. · Via Business Wire · August 6, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that the European Medicines Agency (EMA) has granted Orphan Designation for ADX-2191 (methotrexate intravitreal injection) for the treatment of inherited retinal dystrophies of the rod-dominant phenotype, including retinitis pigmentosa. There are currently no approved drug treatments for patients with most forms of retinitis pigmentosa, a clinical group of rare genetic eye diseases characterized by retinal cell death and loss of vision. Retinitis pigmentosa affects more than one million people worldwide and remains a significant cause of inherited blindness.
By Aldeyra Therapeutics, Inc. · Via Business Wire · July 24, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that the U.S. Food and Drug Administration (FDA) has accepted for review the resubmitted New Drug Application (NDA) for topical ocular reproxalap, a first-in-class investigational new drug candidate, for the treatment of the signs and symptoms of dry eye disease. The FDA assigned a Prescription Drug User Fee Act (PDUFA) target action date of December 16, 2025.
By Aldeyra Therapeutics, Inc. · Via Business Wire · July 17, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced receipt of a Special Protocol Assessment Agreement Letter from the U.S. Food and Drug Administration (FDA) for ADX-2191 (methotrexate injection, USP), an investigational drug candidate, for the treatment of primary vitreoretinal lymphoma (PVRL), a rare and potentially fatal cancer currently lacking FDA-approved therapy.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 26, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune‑mediated and metabolic diseases, today announced the resubmission of a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for topical ocular reproxalap, an investigational new drug candidate, for the treatment of signs and symptoms of dry eye disease. Per FDA agreement, the only new clinical data included in the resubmitted NDA were from the recently completed dry eye chamber trial, which achieved the primary endpoint.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 17, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the 2025 Jefferies Global Healthcare Conference in New York, New York.
By Aldeyra Therapeutics, Inc. · Via Business Wire · May 29, 2025

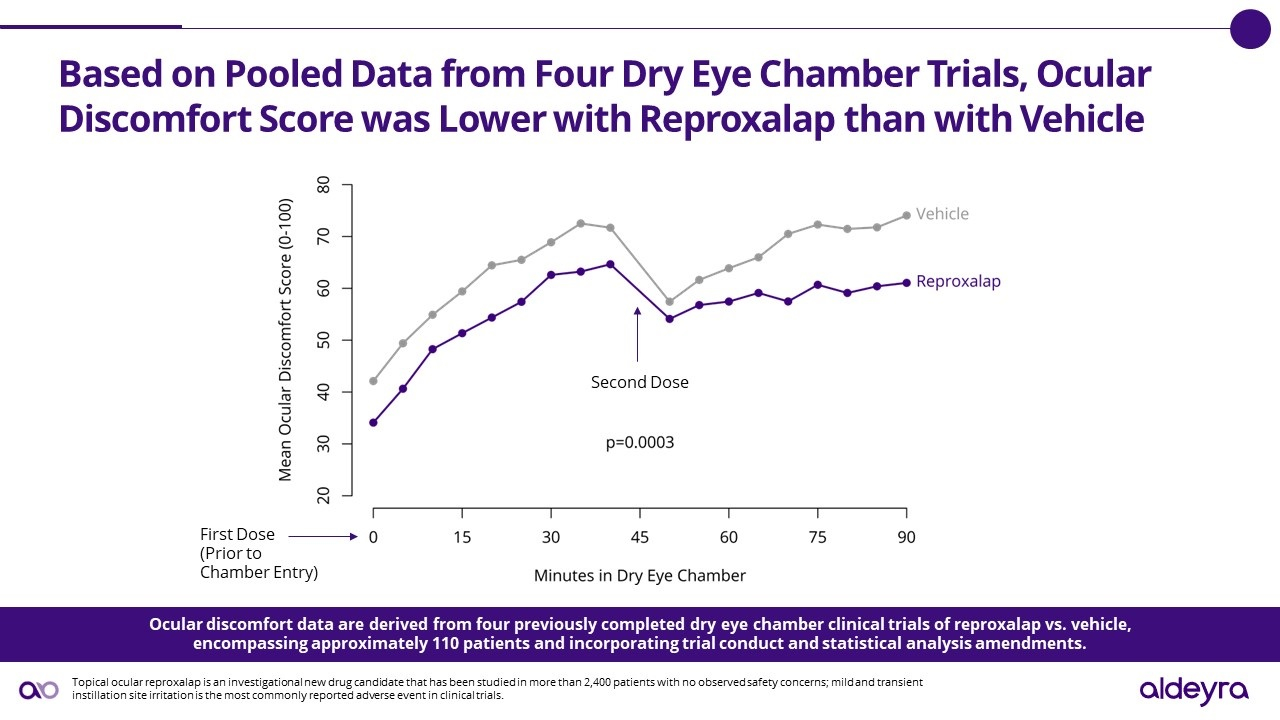
Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced the achievement of the primary endpoint in a Phase 3 randomized, double-masked, vehicle-controlled dry eye chamber trial of 0.25% reproxalap ophthalmic solution, an investigational new drug candidate, for the treatment of dry eye disease. For the prespecified primary endpoint of ocular discomfort, a symptom of dry eye disease, reproxalap (n=58) was statistically significantly superior to vehicle (n=58) on ocular discomfort symptom score (0‑100) from 80 to 100 minutes after chamber entry (LS mean difference [95% confidence interval] ‑6.5 [‑10.5, ‑2.5], P=0.002).
By Aldeyra Therapeutics, Inc. · Via Business Wire · May 5, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced it will host a webcast and conference call on Tuesday, May 6, 2025, at 8:00 a.m. ET to announce topline results from Phase 3 dry eye disease clinical trials of reproxalap. The dial-in numbers are (833) 470-1428 for domestic callers and (404) 975-4839 for international callers. The access code is 127477. A live audio webcast of the conference call will also be accessible from the “Investors & Media” section of Aldeyra's website at ir.aldeyra.com. After the live webcast, the event will remain archived on Aldeyra’s website for 90 days.
By Aldeyra Therapeutics, Inc. · Via Business Wire · May 5, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced the appointment of Chip Clark to the company’s board of directors.
By Aldeyra Therapeutics, Inc. · Via Business Wire · April 17, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced receipt of a Complete Response Letter from the U.S. Food and Drug Administration (FDA) for the resubmission of the New Drug Application (NDA) of reproxalap, an investigational drug candidate, for the treatment of dry eye disease. Although no manufacturing or safety issues with reproxalap were identified, the FDA stated in the letter that the NDA “failed to demonstrate efficacy in adequate and well controlled studies in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted. The letter identified concerns with the data from the trial submitted to the NDA that may have affected interpretation of the results, which the FDA stated may be related to methodological issues, including a difference in baseline scores across treatment arms.
By Aldeyra Therapeutics, Inc. · Via Business Wire · April 3, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the 2025 Leerink Partners Global Healthcare Conference in Miami, Florida.
By Aldeyra Therapeutics, Inc. · Via Business Wire · March 10, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at Oppenheimer’s 35th Annual Healthcare Life Sciences Conference. The conference is being conducted virtually on February 11, 2025.
By Aldeyra Therapeutics, Inc. · Via Business Wire · February 4, 2025

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in the Eyes Wide Open on Ophthalmology Panel at the Citi 2024 Global Healthcare Conference. The conference is being conducted in Miami, Florida December 2-5, 2024.
By Aldeyra Therapeutics, Inc. · Via Business Wire · December 2, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that the U.S. Food and Drug Administration (FDA) has accepted for review the resubmitted New Drug Application (NDA) for topical ocular reproxalap, a first-in-class investigational new drug candidate, for the treatment of the signs and symptoms of dry eye disease. The FDA assigned a Prescription Drug User Fee Act (PDUFA) date of April 2, 2025. In conjunction with the acceptance of the NDA for review, Aldeyra announced the expansion of its exclusive option agreement with AbbVie Inc. (AbbVie).
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 18, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the Jefferies London Healthcare Conference 2024. The conference is being conducted in London, England November 19- 21, 2024.
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 14, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Tomasz Stryjewski, M.D., Chief Medical Advisor - Retina, will present at the Disruptive Innovations Symposium during the Ocular Surgery News (OSN) New York Retina 2024 Meeting, which takes place in New York, New York November 8-10, 2024.
By Aldeyra Therapeutics, Inc. · Via Business Wire · October 31, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced the resubmission of a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for topical ocular reproxalap, an investigational new drug candidate, for the treatment of signs and symptoms of dry eye disease. The resubmission includes previously disclosed positive results from a recently completed dry eye disease symptom trial requested by the FDA following review of the previously submitted NDA, as well as a draft label reflecting acute activity in reducing dry eye symptoms in a dry eye chamber trial, chronic activity in reducing dry eye symptoms in a field trial, and acute activity in reducing ocular redness in two dry eye chamber trials.
By Aldeyra Therapeutics, Inc. · Via Business Wire · October 3, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the H.C. Wainwright 26th Annual Global Investment Conference.
By Aldeyra Therapeutics, Inc. · Via Business Wire · September 5, 2024
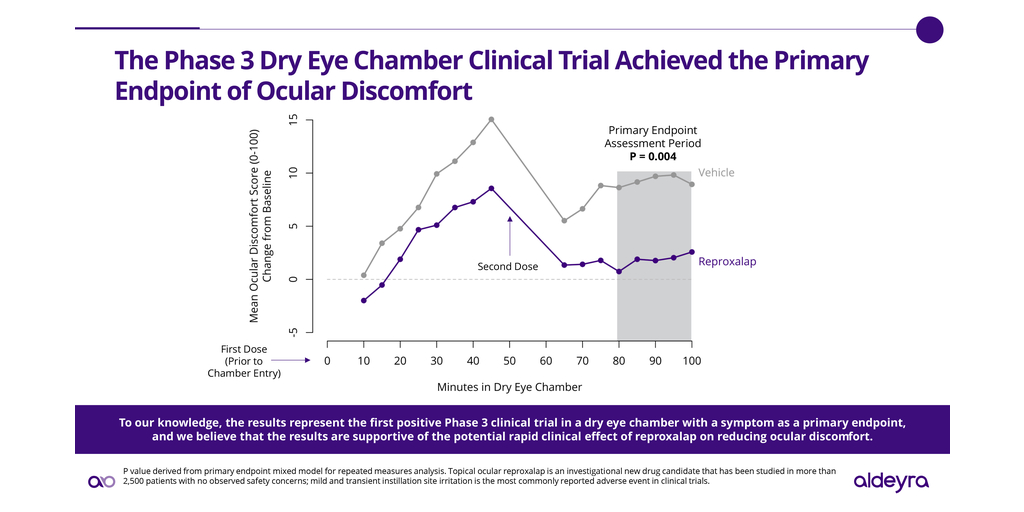
Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced the achievement of the primary endpoint in a Phase 3 randomized, double-masked, vehicle-controlled dry eye chamber clinical trial of 0.25% reproxalap ophthalmic solution, an investigational new drug candidate, for the treatment of dry eye disease. Reproxalap was statistically superior to vehicle for the prespecified primary endpoint of ocular discomfort (P=0.004), a U.S. Food and Drug Administration (FDA)-accepted symptom of dry eye disease.
By Aldeyra Therapeutics, Inc. · Via Business Wire · August 8, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced it will host a webcast and conference call on Thursday, August 8, 2024, at 9:00 a.m. (ET) to provide top-line results from the Phase 3 dry eye disease clinical trial of reproxalap.
By Aldeyra Therapeutics, Inc. · Via Business Wire · August 7, 2024
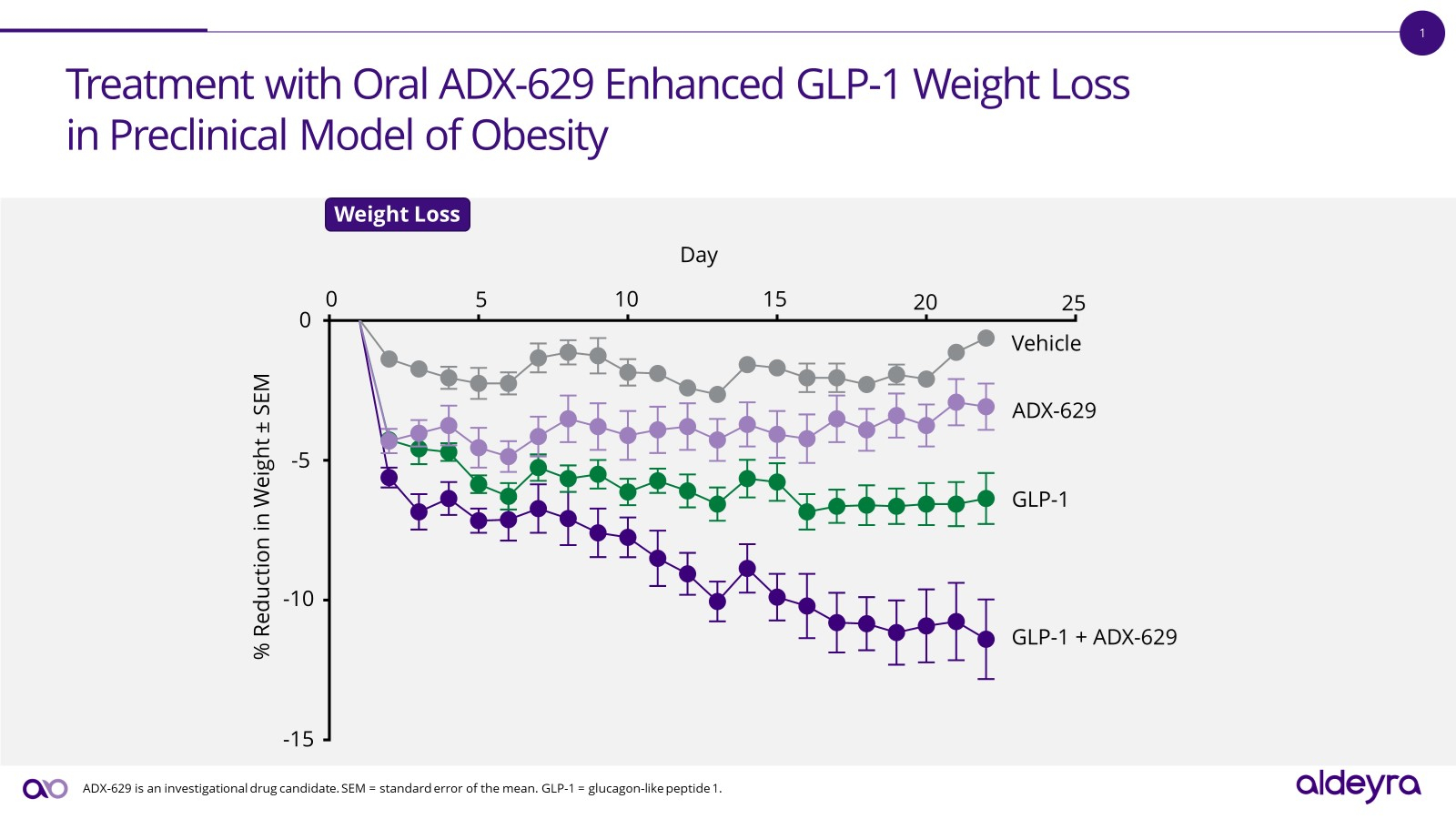
Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced advancement of new RASP modulators and recent preclinical data in obesity in conjunction with an Investor Roundtable scheduled to begin at 8:00 a.m. ET today.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 20, 2024
Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced the completion of enrollment in a Phase 3 dry eye chamber clinical trial of topical ocular 0.25% reproxalap, an investigational RASP modulator, for the treatment of dry eye disease. The trial is designed to enable potential resubmission of a dry eye disease New Drug Application (NDA) in the second half of 2024.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 13, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that the company will host an Investor Roundtable Q&A via webcast on Thursday, June 20, 2024 at 8:00 a.m. ET.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 12, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies for the treatment of immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the Jefferies Global Healthcare Conference.
By Aldeyra Therapeutics, Inc. · Via Business Wire · May 29, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced enrollment of the first patient in a Phase 3 dry eye chamber clinical trial designed to enable a potential resubmission of the New Drug Application (NDA) of topical ocular 0.25% reproxalap, an investigational RASP modulator, for the treatment of dry eye disease.
By Aldeyra Therapeutics, Inc. · Via Business Wire · May 8, 2024
Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) will host the Aldeyra 2024 Research & Development Day with investors and financial analysts in New York City to present recent pipeline developments relating to the RASP modulation platform and ADX-2191 for the treatment of retinitis pigmentosa.
By Aldeyra Therapeutics, Inc. · Via Business Wire · April 25, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies for the treatment of immune-mediated and metabolic diseases, today announced that the company will host a Research & Development Day from 9:00a.m. to 1:00p.m. ET on Thursday, April 25, 2024 in New York City.
By Aldeyra Therapeutics, Inc. · Via Business Wire · April 18, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced the clinical development plan intended to enable resubmission of a New Drug Application (NDA) of topical ocular 0.25% reproxalap, an investigational RASP modulator, for the treatment of dry eye disease to the U.S. Food and Drug Administration (FDA). Following discussions with the FDA, Aldeyra intends to initiate a dry eye chamber clinical trial in the first half of 2024. Contingent on positive results from the planned clinical trial, NDA resubmission is expected in the second half of 2024. Based on FDA guidance, the planned review period for the potential NDA resubmission is expected to be six months.
By Aldeyra Therapeutics, Inc. · Via Business Wire · March 28, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies for the treatment of immune-mediated diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the Oppenheimer 34th Annual Healthcare Life Sciences Conference. The conference will be held virtually on February 13-14, 2024.
By Aldeyra Therapeutics, Inc. · Via Business Wire · February 6, 2024
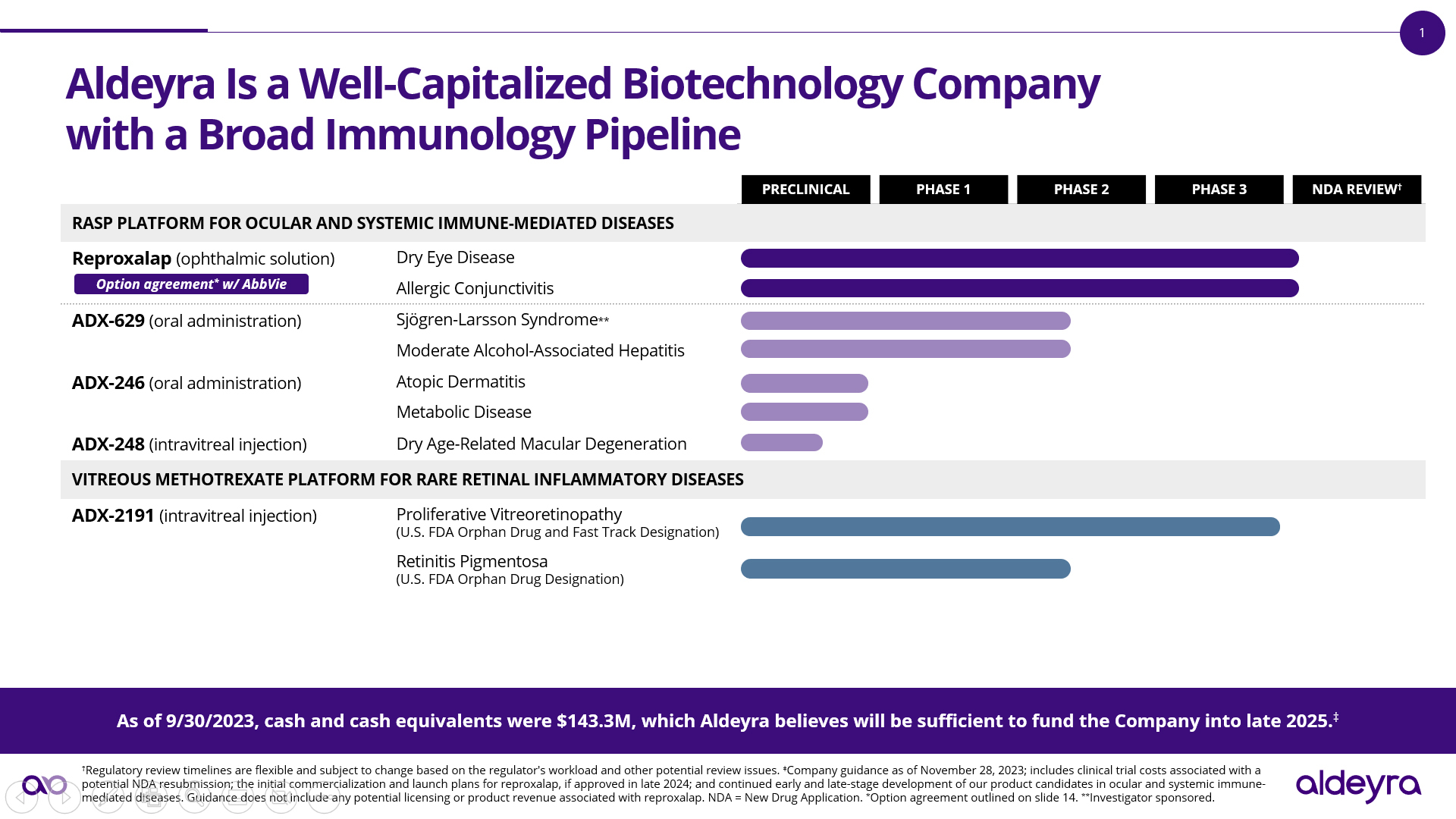
Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced advancement of its RASP modulator platform, including the expected submission to the FDA of a proposed expansion of the Phase 2 clinical trial of the investigational RASP modulator ADX-629 in Sjögren-Larsson Syndrome to include pediatric patients, initiation of a Phase 2 clinical trial of ADX-629 in moderate alcoholic hepatitis, submission of an IND application of the investigational RASP modulator ADX-246 for a Phase 1 clinical trial that is expected to be expanded to include atopic dermatitis patients, anticipated submission of an IND application of the investigational RASP modulator ADX-248 for a Phase 1/2 clinical trial in patients with the dry form of age-related macular degeneration (AMD) and dark adaptation deficit, and initiation of a preclinical program of RASP modulators in metabolic disease.
By Aldeyra Therapeutics, Inc. · Via Business Wire · January 4, 2024

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced positive top-line results from a Phase 2 clinical trial of ADX-629, an investigational RASP modulator, in patients with atopic dermatitis. Relative to baseline, the clinical trial demonstrated statistically significant and clinically relevant improvement in investigator-assessed and patient-reported outcomes across a number of different physiological and psychosocial assessments, including complete resolution of affected body surface area observed in one patient and elimination of itching reported by two patients.
By Aldeyra Therapeutics, Inc. · Via Business Wire · December 19, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced it will host a webcast and conference call on Tuesday, December 19, 2023, at 8:00 a.m. (ET) to provide top-line results from a Phase 2 clinical trial of ADX‑629 in patients with atopic dermatitis.
By Aldeyra Therapeutics, Inc. · Via Business Wire · December 18, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced receipt of a Complete Response Letter from the U.S. Food and Drug Administration (FDA) for the New Drug Application (NDA) of reproxalap, an investigational drug candidate, for the treatment of dry eye disease. Although no safety or manufacturing issues with reproxalap were identified, the FDA stated in the letter that the NDA did not demonstrate “efficacy in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well-controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted.
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 27, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced that it has entered into an exclusive option agreement with AbbVie Inc. (AbbVie).
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 1, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies for the treatment of immune-mediated diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in the SVB Securities Therapeutics Forum. The Forum will be held July 11-12, 2023, in New York City.
By Aldeyra Therapeutics, Inc. · Via Business Wire · July 6, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced positive top-line results from the Phase 2 clinical trial of intravitreal ADX-2191 (methotrexate injection, USP), an investigational drug candidate, in patients with retinitis pigmentosa. Relative to baseline, the clinical trial demonstrated statistically significant improvement in retinal function across a number of different physiological and psychophysical assessments.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 29, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced it will host a webcast and conference call on Thursday, June 29, 2023, at 8:00 a.m. (ET) to provide top-line results from the Phase 2 clinical trial of ADX‑2191 in patients with retinitis pigmentosa.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 28, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced positive top-line results from the Phase 2 clinical trial of orally administered ADX‑629, an investigational new drug, in patients with chronic cough. The clinical trial demonstrated statistically significant reduction in cough frequency following administration of ADX‑629 relative to placebo.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 27, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced it will host a webcast and conference call on Tuesday, June 27, 2023, at 8:00 a.m. (ET) to provide top-line results from the Phase 2 clinical trial of ADX‑629 in patients with chronic cough.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 26, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced it will host a webcast and conference call on Thursday, June 15, 2023, at 8:00 a.m. (ET) to provide top-line results from the Phase 3 INVIGORATE‑2 Trial of reproxalap in allergic conjunctivitis.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 14, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies for the treatment of immune-mediated diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the Jefferies Global Healthcare Conference.
By Aldeyra Therapeutics, Inc. · Via Business Wire · June 2, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today reported recent corporate highlights and financial results for the quarter ended March 31, 2023.
By Aldeyra Therapeutics, Inc. · Via Business Wire · May 4, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced completion of enrollment in the Phase 3 INVIGORATE-2 clinical trial of topical ocular reproxalap, a first-in-class investigational new drug candidate, for the treatment of allergic conjunctivitis.
By Aldeyra Therapeutics, Inc. · Via Business Wire · April 13, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced enrollment of the first patient in the Phase 2 clinical trial of orally administered RASP modulator ADX‑629, an investigational new drug, for the treatment of atopic dermatitis.
By Aldeyra Therapeutics, Inc. · Via Business Wire · April 6, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced the completion of enrollment in the Phase 2 clinical trial of the orally administered RASP modulator ADX-629, an investigational new drug, for the treatment of chronic cough.
By Aldeyra Therapeutics, Inc. · Via Business Wire · March 30, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced the completion of enrollment in the Phase 2 clinical trial of ADX‑2191 (methotrexate injection, USP), an investigational drug candidate, for the treatment of retinitis pigmentosa, a group of rare genetic eye diseases characterized by retinal cell death and loss of vision, for which there is no U.S. Food and Drug Administration (FDA)-approved treatment.
By Aldeyra Therapeutics, Inc. · Via Business Wire · March 16, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today reported recent corporate highlights and financial results for the year ended December 31, 2022.
By Aldeyra Therapeutics, Inc. · Via Business Wire · March 9, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that the U.S. Food and Drug Administration (FDA) accepted for Priority Review the New Drug Application (NDA) for ADX-2191 (methotrexate injection, USP), an investigational drug candidate, for the treatment of primary vitreoretinal lymphoma. The FDA assigned a Prescription Drug User Fee Act (PDUFA) date of June 21, 2023. The FDA noted that no potential filing review issues have been identified.
By Aldeyra Therapeutics, Inc. · Via Business Wire · March 2, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) will host a conference call at 8:00 a.m. ET Thursday, March 9, 2023 to report financial results for the year ended December 31, 2022 and discuss recent corporate highlights.
By Aldeyra Therapeutics, Inc. · Via Business Wire · March 1, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced top-line results from a 12-month, vehicle-controlled, multicenter, parallel-group safety clinical trial of reproxalap, an investigational new drug, in dry eye disease patients. The primary endpoints of treatment-related serious adverse events in ocular safety were not observed in any patient. Ocular safety events were similar across reproxalap and vehicle treatment groups. In a post-hoc analysis, reproxalap was statistically superior to vehicle in improvement from baseline in distance visual acuity, potentially representing the first demonstration of improvement in distance visual acuity with a topically administered therapy.
By Aldeyra Therapeutics, Inc. · Via Business Wire · February 28, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced the advancement of two investigational new drug candidates, ADX‑246 and ADX‑248, to clinical testing, pending completion of U.S. Food and Drug Administration Investigational New Drug (IND) requirements. ADX‑246 and ADX‑248 represent the most recent group of product candidates generated from Aldeyra’s systems-based drug discovery and development engine focused on novel RASP modulators designed to decrease immune responses that lead to disease. Pending completion of the IND requirements, a Phase 1 clinical trial of orally administered ADX‑246 for the treatment of systemic immune-mediated diseases, and a Phase 1/2 clinical trial of intravitreally injected ADX‑248 for the treatment of geographic atrophy, a sight-threatening retinal disease, are expected to initiate in the second half of 2023 or early 2024.
By Aldeyra Therapeutics, Inc. · Via Business Wire · February 23, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced the initiation of Phase 2 clinical trials evaluating the safety and efficacy of ADX‑629, a novel, internally developed, investigational oral RASP modulator, for the treatment of minimal change disease and Sjögren-Larsson Syndrome. Aldeyra also announced the expansion of the minimal change disease clinical trial to encompass idiopathic nephrotic syndrome, a broad group of rare immune-mediated kidney disorders that includes minimal change disease. Additionally, Aldeyra announced the initiation of a Phase 2 clinical trial of ADX‑629 in atopic dermatitis.
By Aldeyra Therapeutics, Inc. · Via Business Wire · February 16, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies for the treatment of immune-mediated diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in a fireside chat at the SVB Securities Global Biopharma Conference. The conference is being conducted in a virtual format February 14-16, 2023.
By Aldeyra Therapeutics, Inc. · Via Business Wire · February 9, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) for topical ocular reproxalap, a first-in-class investigational new drug candidate, for the treatment of the signs and symptoms of dry eye disease. The FDA assigned a Prescription Drug User Fee Act (PDUFA) date of November 23, 2023. The FDA noted that no potential filing review issues have been identified, and that an advisory committee meeting is not currently planned.
By Aldeyra Therapeutics, Inc. · Via Business Wire · February 7, 2023

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced the submission of a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for ADX‑2191 (methotrexate injection, USP), an investigational drug candidate, for the treatment of primary vitreoretinal lymphoma, a rare but potentially fatal cancer with no FDA-approved therapy.
By Aldeyra Therapeutics, Inc. · Via Business Wire · December 21, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced demonstration of target engagement and improvement in the signs of alcohol intoxication in a sequence-randomized, double-masked, placebo-controlled crossover Phase 2 clinical trial of ADX-629, a first-in-class orally administered investigational new drug candidate. Relative to placebo, ADX-629 reduced dermal flushing (P=0.0007), increased Romberg test balance time (P=0.02), and lowered levels of the ethanol RASP metabolite acetaldehyde (P=0.03) following acute exposure to alcohol.
By Aldeyra Therapeutics, Inc. · Via Business Wire · December 13, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that, following the recent receipt of official minutes from its pre-NDA (New Drug Application) meeting with the U.S. Food and Drug Administration (FDA), the Company plans to submit an NDA as soon as the end of 2022 for marketing approval of the investigational drug candidate ADX-2191 for the treatment of primary vitreoretinal lymphoma.
By Aldeyra Therapeutics, Inc. · Via Business Wire · December 1, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced the submission of a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for topical ocular reproxalap, an investigational new drug candidate, for the treatment of signs and symptoms of dry eye disease.
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 29, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies designed to treat immune-mediated diseases, today reported recent corporate highlights and financial results for the quarter ended September 30, 2022.
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 10, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) will host a conference call at 8:00 a.m. ET Thursday, November 10, 2022 to report financial results for the quarter ended September 30, 2022 and discuss recent corporate highlights.
By Aldeyra Therapeutics, Inc. · Via Business Wire · November 3, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that an oral presentation highlighting clinical data from the Phase 3 INVIGORATE allergen chamber trial of reproxalap in allergic conjunctivitis will be presented at the American Academy of Optometry 2022 Annual Meeting, taking place in San Diego October 26-29, 2022.
By Aldeyra Therapeutics, Inc. · Via Business Wire · October 26, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra or the Company) today announced the achievement of the primary endpoint in Part 1 of the Phase 3 GUARD Trial of ADX-2191 (methotrexate injection, USP1) for intravitreal administration, an investigational drug candidate, for the prevention of proliferative vitreoretinopathy (PVR), a rare sight-threatening retinal disease with no approved therapy. ADX-2191 was statistically superior to historical control2 for the prevention of retinal detachment due to PVR over six months (P=0.024).
By Aldeyra Therapeutics, Inc. · Via Business Wire · October 6, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced it will host a webcast and conference call on Thursday, October 6, 2022, at 8:00 a.m. (ET) to report top-line results from Part 1 of the Phase 3 GUARD Trial of ADX-2191 (methotrexate injection, United States Pharmacopeia) for intravitreal administration in patients with proliferative vitreoretinopathy (PVR), a rare, sight-threatening ocular disease with no approved therapy.
By Aldeyra Therapeutics, Inc. · Via Business Wire · October 5, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced that, following the recent receipt of official minutes from its pre-NDA (New Drug Application) meeting with the U.S. Food and Drug Administration (FDA), the Company remains on schedule to submit an NDA in the fourth quarter of 2022 requesting marketing approval of the novel RASP modulator reproxalap, an investigational new drug, for the treatment of dry eye disease.
By Aldeyra Therapeutics, Inc. · Via Business Wire · September 14, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies designed to treat immune-mediated diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer of Aldeyra, will participate in the following investor conferences in September:
By Aldeyra Therapeutics, Inc. · Via Business Wire · September 7, 2022

Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a clinical-stage biotechnology company developing innovative therapies designed to treat immune-mediated diseases, today reported recent corporate highlights and financial results for the quarter ended June 30, 2022.
By Aldeyra Therapeutics, Inc. · Via Business Wire · August 5, 2022
