- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
- Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
- D. Boral Analyst Report on NRXP $34 Price Target.
- Abbreviated New Drug Application (ANDA) is “Substantially Complete” and Received for FDA Review. Assigned Goal Date is July 29, 2026.
- Applied for Use of KETAFREE™ as a Proprietary Product Name as First Preservative-Free Ketamine Formulation.
- Current Worldwide Generic Ketamine Market Estimated at $750 Million Per Year.
- Manufactured Initial Registration Lots of KETAFREE™ and Prepared to Scale Manufacturing to 1 Million Vials Per Month.
- Citizen Petition filed with the FDA Seeking Removal of Benzethonium Chloride Preservative from All Forms of Ketamine Sold in the United States.
- Three Revenue-Generating Facilities in Florida and Expects Six More by Year-End for HOPE Subsidiary Clinics.
- Secured Operating Capital for Drug Development Through July 2026. Expecting Increased Revenue from Clinical Operations.
- Received FDA Grant of Fast Track Designation for NRX-100 in Treatment of Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
- New Real-World Data Support D-Cycloserine (NRX-101 Active Ingredient) in Doubling Effectiveness of Transcranial Magnetic Stimulation.
- First-in-Florida Initiation of One Day (ONE-D) Depression Treatment in Partnership with Ampa Health.
- ONE-D is the First Reported Protocol to Achieve Remission from Treatment-Resistant Depression via Single Day of Treatment, Using an FDA-Cleared Device.
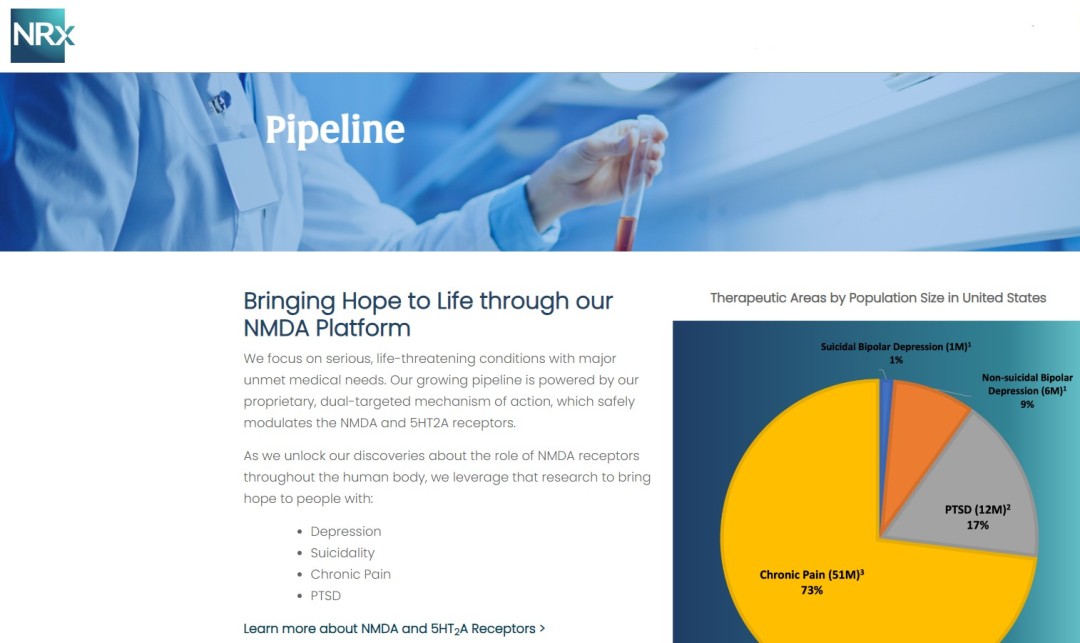
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain.
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
D. Boral has issued an Analyst Report on NRXP with a Buy and $34 Price Target. The full report may be accessed at this direct link: https://www.nrxpharma.com/wp-content/uploads/2025/11/HOPE-Therapeutics-NRXP-Executes-Florida-Roll-out-of-Ampa-O.pdf
US Food and Drug Administration (FDA) Receipt of ANDA for KETAFREE™, a Preservative-Free IV Ketamine
On December 2nd NRXP announced that the U.S. Food and Drug Administration (FDA) has received the Company’s Abbreviated New Drug Application (ANDA) for KETAFREE™, a preservative-free intravenous ketamine formulation. The acknowledgement letter states that the FDA has “made a threshold determination that this NRXP ANDA is substantially complete” and issued a goal date of July 29, 2026 for completion of the final review with potential marketing approval.
Current ketamine products are typically supplied in multi-dose vials that contain a preservative called Benzethonium Chloride (BZT) that is not recognized as safe by FDA and banned from hand cleansers and topical antiseptics.
The current MAHA initiatives have called for review and removal of toxic substances from foods, drugs, and vaccines and NRXP KETAFREE™, designed to align with those priorities, is intended for all currently approved ketamine indications and is manufactured in the United States, supporting national efforts to strengthen the domestic supply of critical medicines.
KETAFREE™ is separate from the NRXP New Drug Application for NRX-100, which is being developed as an innovative drug for the treatment of suicidal depression and has received Fast Track designation from the FDA.
NRXP continues to advance other elements of its pipeline and its development of HOPE Therapeutics clinics, a report being presented at the December 3rd Noble Securities NOBLECON conference and available on the Company’s website.
Third Quarter 2025 Corporate Update
On November 17th NRXP announced financial results for the quarter ended September 30, 2025, and provided a corporate update.
Dr. Jonathan Javitt, CEO of NRXP stated, “In 2025 we have advanced each of our corporate objectives and entered into revenue-generating activity for the first time. For NRX-100 in suicidal depression, we received an expanded Fast Track designation, opened an Expanded Access program and enhanced our regulatory package. Additionally, FDA granted our Suitability Petition for a single patient, preservative free ketamine strength and we have received validation that our ANDA filing is on track with no major deficiencies. In parallel, the Real World Data demonstrating a doubling of antidepressant and antisuicidal effect of Transcranial Magnetic Stimulation (TMS) when D-cycloserine is added creates a new and significant indication for NRX-101 that has potential for approval, if confirmed in an additional phase 3 trial. For HOPE, we continue to execute on building our delivery platform of three active facilities in Florida, with three more planned by year-end. Dr. Rebecca Cohen and LTC Charles Paul, RN (US Army, Ret.) are assembling a network of best-in-class interventional psychiatrists to meet the needs of people, including active duty military, first responders, and veterans, across Florida and beyond.”
Key Research and Development and Corporate Activities:NRXP is pursuing two paths to market for NRX-100: a distinct innovative pathway via a New Drug Application (NDA) under FDA Fast Track designation to develop NRX-100 for suicidal ideation in depression, including bipolar depression, and a generic pathway via an Abbreviated New Drug Application (ANDA) to supply a preservative-free ketamine (KETAFREE™) into the existing ketamine market.
Recent correspondence from the FDA suggests that the latter pathway is on track for a Q2 2026 GDUFA date. The current generic ketamine market is estimated at approximately $750 million. By contrast, the innovative ketamine-based product SPRAVATO® is expected to generate over $1.6 billion in 2025 sales, although its labeling states that effectiveness in preventing suicide or reducing suicidal ideation has not been demonstrated. This highlights a differentiated opportunity for NRXP NRX-100, specifically in suicidal ideation. A New Drug Application (“NDA”) for NRX-100 for suicidal depression, originally initiated during the fourth quarter of 2024, is expected to be completed in the fourth quarter 2025 with the addition of Real World Efficacy Data drawn from more than 60,000 patients treated for depression with intravenous ketamine compared to 6,000 patients treated with intranasal S-ketamine to be submitted as part of the NDA. An interim analysis drawn from the first 20,000 patients suggests that IV ketamine may have a more rapid onset of action and larger magnitude of effect than nasal S-ketamine. The Company has applied to receive a Commissioner’s National Priority Voucher (CNPV), which could significantly reduce review time.
NRXP has now manufactured multiple commercial lots of NRX-100 and KETAFREE™ with ongoing stability data supporting a room temperature shelf life of three years.
Third Quarter 2025 Financial Results
NRXP third quarter 2025 financial results were released on November 17th via public disclosure media and on the Company's website at https://ir.nrxpharma.com/. NRXP management also hosted a conference call on the same day. A live webcast of the conference call will be available on the NRXP website at https://ir.nrxpharma.com/events.
First-in-Florida Initiation of One Day (ONE-D) Depression Treatment in Partnership with Ampa Health
On November 10th NRXP announced initiation of patient care with for treatment-resistant depression with the Ampa one day (ONE-D) protocol. NRXP is the first to deploy the Ampa technology in Florida and one of the first deployments nationwide. The Ampa device differs from other Transcranial Magnetic Stimulation (TMS) treatments in that the peer-reviewed literature has reported a high rate of success (87% response and 72% remission) in nonrandomized trials when a single day of TMS treatment is combined with physician-prescribed D-cycloserine and lisdexamfetamine (note that neither of the drugs reported in the published results is FDA-approved for the stated indication).
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: Send Email
Phone: 484 254 6134
Address:1201 Orange Street Suite 600
City: Miami
State: Florida
Country: United States
Website: https://www.nrxpharma.com/

